��Ŀ����
13����֪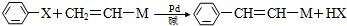 ��XΪ±ԭ�ӣ�MΪ������������ȡ�����ȣ������л���A�ϳ�G���㶹�أ���·�����£�
��XΪ±ԭ�ӣ�MΪ������������ȡ�����ȣ������л���A�ϳ�G���㶹�أ���·�����£�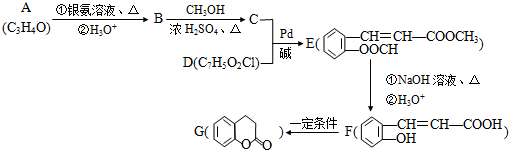
��ش��������⣺
��1��������C�еĺ��������ŵ�������������F��G�ķ�Ӧ������������Ӧ��ȡ����Ӧ����
��2��д��B��C��ѧ����ʽCH2=CH-COOH+CH3OH$\frac{\underline{\;ŨH_{2}SO_{4}\;}}{��}$CH2=CH-COOCH3+H2O��
��3��������D�Ľṹ��ʽΪ
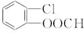 ��
����4��д��ͬʱ��������������F��һ��ͬ���칹��Ľṹ��ʽ
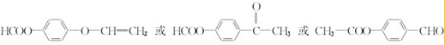 ��
�������г������⣬��������״�ṹ��
���������������ڶ�λ��ȡ������
���ܷ���ˮ�ⷴӦ�������������Na��Ӧ��
�����������Ƶ�Cu��OH��2�����ʵ���֮��1��2������Ӧ��
��5�������㶹�أ�
 �������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨
�������㶹�ص����Ʒ����������㶹�غ�����һ��ͬ���칹�壨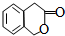 ����Ҫ�õ����Լ��У�NaOH��Һ��ϡ������Ȼ�����Һ��
����Ҫ�õ����Լ��У�NaOH��Һ��ϡ������Ȼ�����Һ����6����֪��
 ��R��R��Ϊ���������Ա�����ϩ��CH2=CH-CH3��Ϊԭ�ϣ��Ʊ�
��R��R��Ϊ���������Ա�����ϩ��CH2=CH-CH3��Ϊԭ�ϣ��Ʊ� 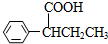 �ĺϳ�·������ͼ���£��벹����������ͼ�����Լ���ѡ����
�ĺϳ�·������ͼ���£��벹����������ͼ�����Լ���ѡ����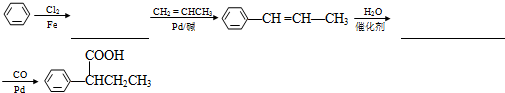
���� ���л���A�ϳ�G���㶹�أ���·�߿�֪��A�ܷ���������Ӧ����AΪCH2=CHCHO��BΪCH2=CHCOOH��B��״�����������Ӧ��CΪCH2=CHCOOCH3�������֪��Ϣ��֪C+D��E����E�Ľṹ��֪��DΪ ��E��F����ˮ�⣬F��G����������Ӧ��
��E��F����ˮ�⣬F��G����������Ӧ��
��1��C�к�C=C��-COOC-��F��G����������Ӧ��
��2��BΪCH2=CHCOOH��B��״�����������Ӧ����C��CΪCH2=CHCOOCH3��
��3��������������֪��DΪ ��
��
��4��F��ͬ���칹����������г������⣬��������״�ṹ�����������������ڶ�λ��ȡ���������ܷ���ˮ�ⷴӦ�����������Na��Ӧ�������������Ƶ�Cu��OH��2�����ʵ���֮��1��2������Ӧ����-COOC-��-CHO��HCOOC-��-C-O-C-CH=CH2��HCOOC-��-COCH3��
��5�������㶹�ص�ˮ������к����ӽṹ�����ñ��ӵ����ʼ���
��6���Ա�����ϩ��CH2�TCHCH3��Ϊԭ���Ʊ�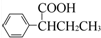 ���ȷ�������ȡ�����ٽ����Ϣ��֪�������ȱ����CH2�TCHCH3��Ӧ��Ȼ����ˮ�����ӳɣ������CO��Pb��Ӧ���ɲ��
���ȷ�������ȡ�����ٽ����Ϣ��֪�������ȱ����CH2�TCHCH3��Ӧ��Ȼ����ˮ�����ӳɣ������CO��Pb��Ӧ���ɲ��
��� �⣺���л���A�ϳ�G���㶹�أ���·�߿�֪��A�ܷ���������Ӧ����AΪCH2=CHCHO��BΪCH2=CHCOOH��B�������������Ӧ��CΪCH2=CHCOOCH3�������֪��Ϣ��֪C+D��E����E�Ľṹ��֪��DΪ ��E��F����ˮ�⣬F��G����������Ӧ��
��E��F����ˮ�⣬F��G����������Ӧ��
��1��C�к�C=C��-COOC-������������Ϊ������F��G����������Ӧ��ȡ����Ӧ�����ʴ�Ϊ��������������Ӧ��ȡ����Ӧ����
��2��BΪCH2=CHCOOH��B��״�����������Ӧ����C��CΪCH2=CHCOOCH3����ѧ����ʽΪ��CH2=CH-COOH+CH3OH $\frac{\underline{\;ŨH_{2}SO_{4}\;}}{��}$ CH2=CH-COOCH3+H2O��
�ʴ�Ϊ��CH2=CH-COOH+CH3OH $\frac{\underline{\;ŨH_{2}SO_{4}\;}}{��}$ CH2=CH-COOCH3+H2O��
��3��������������֪��DΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��4��F��ͬ���칹����������г������⣬��������״�ṹ�����������������ڶ�λ��ȡ���������ܷ���ˮ�ⷴӦ�����������Na��Ӧ�������������Ƶ�Cu��OH��2�����ʵ���֮��1��2������Ӧ����-COOC-��-CHO��HCOOC-��-C-O-C-CH=CH2��HCOOC-��-COCH3������������ͬ���칹��Ϊ ��
��
�ʴ�Ϊ�� ��
��
��5�������㶹�ص�ˮ������к����ӽṹ�����ñ��ӵ����ʼ��������Լ���NaOH�⣬��ѡ��ϡ�����к������ԣ�ѡ�Ȼ�����Һ����ɫ���鱽�ӣ�
�ʴ�Ϊ��ϡ������Ȼ�����Һ��
��6���Ա�����ϩ��CH2�TCHCH3��Ϊԭ���Ʊ�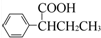 ���ȷ�������ȡ�����ٽ����Ϣ��֪�������ȱ����CH2�TCHCH3��Ӧ��Ȼ����ˮ�����ӳɣ������CO��Pb��Ӧ���ɲ���ϳ�·������ͼΪ
���ȷ�������ȡ�����ٽ����Ϣ��֪�������ȱ����CH2�TCHCH3��Ӧ��Ȼ����ˮ�����ӳɣ������CO��Pb��Ӧ���ɲ���ϳ�·������ͼΪ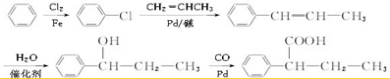 ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϡ��л���Ľṹ�����ʣ�ע��������Ϣ���ϳ�����ͼ�ƶϳ��������ǽ����Ĺؼ���ע���л���Ľṹ�仯����Ŀ�Ѷ��еȣ�

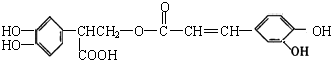
| A�� | �Ե�������ʹ���Ը�����ؼ�������Ȼ�̼��Һ��ɫ�����ڷ����� | |
| B�� | �Ե�����ķ���ʽΪC18H15O8�����ڼ��������µ�ˮ�ⷴӦ��������Ӧ | |
| C�� | �Ե���������к���5�ֹ����� | |
| D�� | 1mol�Ե������H2��Ӧ����������7molH2 |
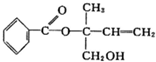
| A�� | X�ķ���ʽΪC5H10O2���ò������������ͬ���칹����5�� | |
| B�� | X��һ���������ܷ����ӳɡ��Ӿۡ�ȡ������ȥ�ȷ�Ӧ | |
| C�� | ��Ni�������������£�1 mol X���ֻ����1 mol H2�ӳ� | |
| D�� | �������Ը��������Һ���ֱ���X |
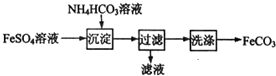
| A�� | ������KSCN��Һ����FeSO4��Һ�Ƿ���� | |
| B�� | ������������CO2����ų� | |
| C�� | ���������ij��ò����������ձ���©���Ͳ����� | |
| D�� | ��ƷFeCO3�ڿ����и��·ֽ�ɵõ�������FeO |
| A�� | ������������ | |
| B�� | ��ˮ�¶����ߣ���ط�Ӧ���ʿ��ܼӿ� | |
| C�� | �����Ƴɷ���������״�ṹ��Ŀ���������������Ϻ������Ӵ���� | |
| D�� | �����缫��ӦʽΪO2+4H++4e-=2H2O |

| A�� | ��Һ��һ��û��Ba2+��NO3-��CO32- | |
| B�� | ��Һ��һ������H+��NH4+��Fe2+��SO42-��Cl- | |
| C�� | ����HΪһ��Al��OH��3��BaCO3�Ļ���� | |
| D�� | ����ҺX�����Ϊ100 mL�����������112 mL����A����X��c��Fe2+��=0.05mol•L-1 |
| A�� | 9�� | B�� | 16�� | C�� | 32�� | D�� | 64�� |
| A�� | 6.4g��S2��S4��S8��ɵĻ������������ԭ����Ϊ0.2NA | |
| B�� | ��100ml 4mol/L������8.7g MnO2��������ȡ����4.48L | |
| C�� | һ���¶��£�0.1L 0.1mol/L��CH3COOH��Һ��1L 0.01mol/L ��CH3COOH��Һ����CH3COO-����Ŀ��Ϊ0.01NA | |
| D�� | һ�������£���1mol N2��3 mol H2��ϣ���ַ�Ӧ��ת�Ƶĵ�����Ϊ6 NA |
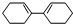 c��
c��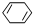 d��
d��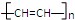
 +
+ $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$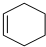 ��Ҫ�Ʊ�
��Ҫ�Ʊ�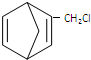 ��������ṹ��ԭ�Ͽ�����
��������ṹ��ԭ�Ͽ�����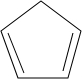 ��
��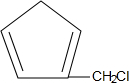 ����д�ṹ��ʽ��
����д�ṹ��ʽ��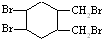 �ĺϳ�·�ߣ��������Լ���ѡ����
�ĺϳ�·�ߣ��������Լ���ѡ����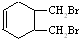 $\stackrel{Br_{2}}{��}$
$\stackrel{Br_{2}}{��}$