��Ŀ����
1����ѧ�кܶ���ɣ����ʣ����������÷�Χ�����и������Ƴ��Ľ�����ȷ���ǣ�������| ѡ�� | ���ɣ������ʣ� | �� �� |
| A | ����Ԫ����������ϼ۵��������� | ��V��A��Ԫ��������۶���+7 |
| B | SO2��ʪ���Cl2����Ư���� | ����Ϻ�Ư���Ը�ǿ |
| C | ��ʪ��ͭ��Ũ���ᷴӦ������ȡNO2 | ����������Ũ���ᷴӦҲ������ȡNO2 |
| D | ��ǿ�������ȡ������ | CO2ͨ��NaClO��ҺҺ��������HC10 |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
���� A����V��A��Ԫ�ط������ۣ�
B�����������������ˮ��Һ�лᷢ��������ԭ��Ӧ��
C���������Ũ���ᷢ���ۻ�����
D��̼������Աȴ����������ǿ��
��� �⣺A����V��A��Ԫ�ط������ۣ����۲���ȷ����A����
B�������ʵ����Ķ��������������ˮ��Һ�лᷢ��������ԭ��Ӧ������������ᣬ���پ���Ư���ԣ���B����
C���������Ũ���ᷢ���ۻ��������Գ���������Ũ���ᷴӦҲ������ȡNO2����C����
D��̼������Աȴ����������ǿ������CO2ͨ��NaClO��ҺҺ��������HC10����D��ȷ��
��ѡD��
���� ���⿼�������ʵ����ʷ����֪ʶ���Ѷ����У�����ʱҪע��һ���������������Ĺ�ϵ���Լ����������õ������ȣ�

��ϰ��ϵ�д�
�����Ŀ
11��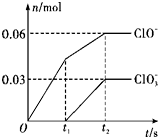 ��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
 ��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
��һ������Cl2ͨ��һ��Ũ�ȵĿ��Լ���Һ�У�����ǡ����ȫ��Ӧ����֪��Ӧ���̷��ȣ����������������ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-�������ӵ����ʵ�����n���뷴Ӧʱ�䣨t���ı仯��ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | Cl2�Ϳ��Լ���Һ�ڲ�ͬ�¶��¿��ܷ�����ͬ��Ӧ | |
| B�� | ��Ӧ��ת�Ƶ�����Ϊ��0.42 NA | |
| C�� | ԭ���Լ���Һ��KOH�����ʵ���Ϊ0.3 mol | |
| D�� | ��������Cl-�����ʵ���Ϊ0.21 mol |
9��25��ʱ�����и���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | ���ʹ��Na2CO3����Һ�У�2c��Na+��=c��CO32-��+c��HCO3-��+c��H2CO3�� | |
| B�� | 1mol/L�ģ�NH4��2SO4��Һ�У�c��NH4+����c��SO42-����c��H+����c��OH-�� | |
| C�� | 0.10mol/L��������Һ�У�c��Na+��+c��H+��=c��CH3COO-��+c��OH-�� | |
| D�� | �������pH=3������ʹ����к��������Ƶ����ʵ�����ͬ |
16��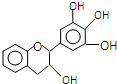 ���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������
���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������
 ���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������
���豶���������к��в��ӣ�����ûʳ�Ӷ����أ�EGC���Ľṹ��ͼ��ʾ������EGC��������������ȷ���ǣ�������| A�� | ���л���ķ���ʽ��C15H13O5 | |
| B�� | 1mol EGC��4molNa�����������Ϊ44.8L | |
| C�� | ����������Ӧ��ȡ����Ӧ���ѷ����ӳɷ�Ӧ | |
| D�� | ���������е�ԭ�ӹ��� |
6��������Ԫ��A��B��C��D��ԭ������������������ԭ�ӵ�����������֮��Ϊ16��A��Dͬ���壬B+��A2-������ͬ�ĵ��Ӳ�ṹ��Cԭ�ӵ���������������Aԭ��������������һ�룬������������ȷ���ǣ�������
| A�� | B2A2��B2A���������ӵĸ�������ͬ | |
| B�� | ԭ�Ӱ뾶�Ĵ�С˳��r��D����r��C����r��B����r��A�� | |
| C�� | D�ļ���̬�⻯������ȶ��Ա�A��ǿ | |
| D�� | Ԫ��C�ĵ�����һ�ָ�Ӳ�ȡ����۵�Ľ��� |
13��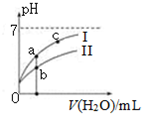 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��ͼ��ʾ�����£�ϡ��CH3COOH��HClO�������ϡ��Һʱ����ҺpH���ˮ���ı仯������˵����ȷ���ǣ�������
 ��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����
��֪���±�Ϊ25��ʱijЩ����ĵ���ƽ�ⳣ����| CH3COOH | HClO | H2CO3 |
| Ka=1.8��10-5 | Ka=3.0��10-8 | Ka1=4.4��10-7Ka2=4.7��10-11 |
| A�� | ��ͬŨ�ȵ�CH3COONa��NaClO�Ļ����Һ�У�������Ũ�ȵĴ�С��ϵ�ǣ�c��Na+����c��ClO-����c��CH3COO-����c��OH-����c��H+�� | |
| B�� | ��NaClO��Һ��ͨ������������̼�����ӷ���ʽΪ��ClO-+CO2+H2O=HClO+CO32- | |
| C�� | ͼ����a��c���㴦����Һ��$\frac{c��{R}^{-}��}{c��HR��•c��O{H}^{-}��}$��ȣ�HR����CH3COOH��HClO�� | |
| D�� | ͼ����a�������Ũ�ȴ���b�������Ũ�� |
10�� �״���һ����Ҫ�Ļ���ԭ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ�
��1����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽCH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-725.8kJ/mol��
��2���״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ�����䷴ӦΪ��
CH3OH ��g��+H2O ��g��?CO2��g��+3H2��g����H=-72.0kJ/mol
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��{c}^{3}��{H}_{2}��}{c��C{H}_{3}OH��c��{H}_{2}O��}$��
�����д�ʩ����ʹƽ��ʱ$\frac{n��C{H}_{3}OH��}{n��C{O}_{2}��}$��С���ǣ�˫ѡ��CD��
A��������� B�����ݳ���He��g����ʹ��ϵѹǿ����
C����H2��g������ϵ�з��� D�������ٳ���1molH2O��g��
��3���״����������ɼ��ᣬ�ڳ�������0.1000mol/L NaOH��Һ�ζ�20.00mL 0.1000mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V��NaOH����
�������������=���� 20.00mL��
��4�����ü״�ȼ�����Ϊȼ�ϵ�أ���ͼ��ʾ�����缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
��5���ϳɼ״�����Ҫ��ӦΪ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/molԭ�����ļӹ������г�������һЩCO2��Ϊ���о��¶ȼ�CO2�����Ը÷�Ӧ��Ӱ�죬��CO2��CO��H2�Ļ������Ϊԭ����һ�������½���ʵ�飮ʵ�����ݼ��±���
�ɱ������ݿɵó�������ۣ�
����һ����һ�������£���Ӧ�¶�Խ�ߣ�����CH3OH��̼ת����Խ�ߣ�
���۶���ԭ����������CO2������������ɼ״���̼ת���ʣ�CO2�����������ɼ״���̼ת�����ֽ��ͣ�
 �״���һ����Ҫ�Ļ���ԭ�ϣ�
�״���һ����Ҫ�Ļ���ԭ�ϣ���1����֪����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=-1275.6kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽCH3OH��l��+$\frac{3}{2}$O2��g��=CO2��g��+2H2O��l����H=-725.8kJ/mol��
��2���״���ˮ�����������ɻ�������Դ�����й㷺��Ӧ��ǰ�����䷴ӦΪ��
CH3OH ��g��+H2O ��g��?CO2��g��+3H2��g����H=-72.0kJ/mol
�ٸ÷�Ӧ��ƽ�ⳣ������ʽΪK=$\frac{c��C{O}_{2}��{c}^{3}��{H}_{2}��}{c��C{H}_{3}OH��c��{H}_{2}O��}$��
�����д�ʩ����ʹƽ��ʱ$\frac{n��C{H}_{3}OH��}{n��C{O}_{2}��}$��С���ǣ�˫ѡ��CD��
A��������� B�����ݳ���He��g����ʹ��ϵѹǿ����
C����H2��g������ϵ�з��� D�������ٳ���1molH2O��g��
��3���״����������ɼ��ᣬ�ڳ�������0.1000mol/L NaOH��Һ�ζ�20.00mL 0.1000mol/L ������Һ�����У������Һ��pH=7ʱ�������ĵ�V��NaOH����
�������������=���� 20.00mL��
��4�����ü״�ȼ�����Ϊȼ�ϵ�أ���ͼ��ʾ�����缫��ӦʽΪCH3OH-6e-+8OH-=CO32-+6H2O��
��5���ϳɼ״�����Ҫ��ӦΪ��CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/molԭ�����ļӹ������г�������һЩCO2��Ϊ���о��¶ȼ�CO2�����Ը÷�Ӧ��Ӱ�죬��CO2��CO��H2�Ļ������Ϊԭ����һ�������½���ʵ�飮ʵ�����ݼ��±���
| CO2%-CO%-H2% ����������� | 0-30-70 | 2-28-70 | 4-26-70 | 8-22-70 | ||||||||
| ��Ӧ�¶�/�� | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 | 225 | 235 | 250 |
| ����CH3OH��̼ת���ʣ�%�� | 4.9 | 8.8 | 11.0 | 36.5 | 50.7 | 68.3 | 19.0 | 33.1 | 56.5 | 17.7 | 33.4 | 54.4 |
����һ����һ�������£���Ӧ�¶�Խ�ߣ�����CH3OH��̼ת����Խ�ߣ�
���۶���ԭ����������CO2������������ɼ״���̼ת���ʣ�CO2�����������ɼ״���̼ת�����ֽ��ͣ�
11�����з���ʽ��ʾ���л����ͬ���칹�干�����ֵ��ǣ������������칹����������
| A�� | C3H7Cl | B�� | C3H6Cl2 | C�� | C3H5Cl3 | D�� | C3HCl7 |