��Ŀ����
1���������ӷ���ʽ��ȷ���ǣ�������| A�� | �����������������������Һ��Ӧ��Fe��OH��3+3H+=Fe3++3H2O | |
| B�� | ����������Һ�м������������Һ��2Fe2++H2O2+2H+=2Fe3++2H2O | |
| C�� | NaHCO3��Һ�м�������ʯ��ˮ��HCO3-+Ca2++OH-=CaCO3��+H2O | |
| D�� | ��NaAlO2��Һ��ͨ������CO2��2AlO2-+CO2+3H2O�T2Al��OH��3��+CO32- |
���� A�������������ܹ��������������ɵ��ʵ⣻
B��˫��ˮ����ǿ�������ԣ��ܹ����������������������������ӣ�
C�����߷�Ӧ����̼��ơ��������ƺ�ˮ��
D��������̼��������̼�����ƺ�����������
��� �⣺A�������������������������Һ��Ӧ�����ӷ���ʽ��2Fe��OH��3+2I-+6H+�T2Fe2++I2��+6H2O����A����
B������������Һ�м������������Һ�����ӷ���ʽ��2Fe2++H2O2+2H+=2Fe3++2H2O����B��ȷ��
C��NaHCO3��Һ�м�������ʯ��ˮ�����ӷ���ʽ��2HCO3-+Ca2++2OH-=CaCO3��+2H2O+CO32-����C����
D����NaAlO2��Һ��ͨ������CO2�����ӷ���ʽ��AlO2-+CO2+2H2O�TAl��OH��3��+HCO3-����D����
��ѡ��B��
���� ���⿼�������ӷ���ʽ����д����ȷ��Ӧʵ���ǽ���ؼ���ע�����ӷ���ʽӦ��ѭ����ʵ����ѭԭ�Ӹ�����������غ���ɣ�ע�ⷴӦΪ�����Է�ӦӰ�죬��Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ
12�����к͵ζ��ⶨij�����Ũ�ȣ��������£�����ƽ�ӵ�20gNaOH�����1000ml����Һ�������ⶨ��ԼŨ��Ϊ1mol/L������50mL��������õ�NaOH��Һֱ�������ⶨ����ʵ���������Ϊһ�Σ�Լ0.05mL������ô��ȡNaOH�������ƽ��ȷ�����������������ĸ��ȽϺ�����������
| A�� | 0.1g | B�� | 0.01g | C�� | 0.001g | D�� | 0.0001g |
16�����з�Ӧ�����ӷ���ʽ��д��ȷ���ǣ�������
| A�� | �ð�ˮ��������SO2���壺NH3•H2O+SO2��NH4++HSO3- | |
| B�� | FeI2��Һ��ͨ��Cl2��Fe2+ǡ����ȫ��������2Fe2++Cl2��2Fe3++2Cl- | |
| C�� | ̼�������Һ�м����������������Һ��HCO3-+OH-��CO32-+H2O | |
| D�� | ��Ca��ClO��2��Һ��ͨ��������CO2���壺ClO-+CO2+H2O��HClO+HCO3- |
10��ʵ�����ڳ�����0.1mol•L-1ijһԪ�BOH��pH��13��0.1mol•L-1��ijһԪ��$\frac{{H}^{+}}{O{H}^{-}}$=1012��HA����Һ�н�������Һ�������Ϻ���Һ������Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | [A-]��[B+]��[H+]��[OH-] | B�� | [B+]��[A-]��[H+]��[OH-] | C�� | [B+]��[A-]��[OH-]=[H+] | D�� | [A-]��[B+]��[OH-]��[H+] |
11�������£����и�������һ�����Դ���������ǣ�������
| A�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=10-4����Һ�У�Na+��SiO32-��SO32-��K+ | |
| B�� | ����Al�ܷų�H2����Һ�У�Cl-��Mg2+��NO3-��K+ | |
| C�� | ��ˮ�������c��OH-��=10-13mol•L-1����Һ�У�ClO-��Na+��SO32-��Cl- | |
| D�� | pH=1����Һ�У�Mg2+��Br-��K+��AlO2- |
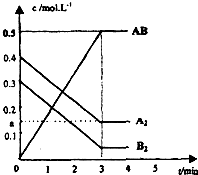 ��һ���ݻ�Ϊ2L���ܱ������У�����0.8mol��A2�����0.6molB2���壬һ�������·������·�Ӧ��A2��g��+B2��g��?2AB��g����H��0����Ӧ�и����ʵ�Ũ����ʱ��ı仯�����ͼ��ʾ��
��һ���ݻ�Ϊ2L���ܱ������У�����0.8mol��A2�����0.6molB2���壬һ�������·������·�Ӧ��A2��g��+B2��g��?2AB��g����H��0����Ӧ�и����ʵ�Ũ����ʱ��ı仯�����ͼ��ʾ�� �廯������ʯ���꾮��Ҳ���������廯識�����ֽ��������������ȣ���ҵ�����ú�ˮ�ͱ���Ϊԭ���Ʊ�CaBr2•2H2O����Ҫ�������£�
�廯������ʯ���꾮��Ҳ���������廯識�����ֽ��������������ȣ���ҵ�����ú�ˮ�ͱ���Ϊԭ���Ʊ�CaBr2•2H2O����Ҫ�������£�