��Ŀ����
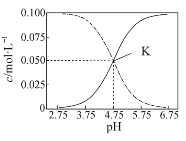
����Ŀ���ⶨ����ͭ���壨CuSO4XH2O ����Xֵ��ʵ��������£�
![]()
��1��������ʵ�����õ��ļ����������������Ӧ������ȷ����___________��
��2�������ա�ʱ��Դѡ�õ��Ǿƾ��ƶ����Ǿƾ���ƣ�������_____________________������ȴ������_______________��(����������)��
��3�� �����ء�������Ŀ����_________________________________________________��
�жϡ����ء���������_________________________________________________��
��4��������ijѧ��ʵ���һ�����ݣ�����ɼ���
�������� | �����뾧�������� | ���Ⱥ���������������� |
11.721g | 22.692g | 18.631g |
X=__________________��(��ȷ��0.01)��ʵ����������_________________��(����С�����һλ)
��5�����ʵ���в�������ԭ�������__________����ɵġ�
a������ͭ�����к��в��ӷ������ʡ��� b���ڼ��ȹ��̷����к�ɫ��������
c������ʱ�о���ɽ����������������� d������ʧˮ��¶���ڿ�������ȴ
���𰸡�ac �ƾ�����¶�̫������ʹ����ͭ�ֽ� ������ ȷ������ͭ����ʧȥȫ���ᾧˮ �������γ��������������0.001g 5.22 +4.4% b��c
��������
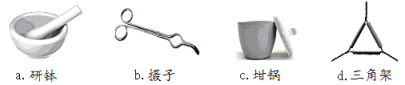
��1������������ͼ��֪��aΪ�в���bΪ����ǯ��cΪ������dΪ�����ǣ��������Ӧ������ȷ����ac���ʴ�Ϊ��ac��
��2�������ա�ʱ��Դѡ�õ��Ǿƾ��ƶ����Ǿƾ���ƣ��ƾ���ƶԾ������ʱ�¶ȹ��ߣ�����ͭ��ֽ⣬Ϊ��ֹ��ˮ��ͭ����ȴ���� �����տ����е�ˮ�ݣ�Ӱ��ʵ��������ȴӦ�ڸ������У��ʴ�Ϊ���ƾ�����¶�̫������ʹ����ͭ�ֽ⣻��������
��3����������������Ŀ�ģ�ȷ������ͭ������ȫʧȥ�ᾧˮ����֤����ʵ���ȷ�ԣ��ж��������������ݣ��������γ��������������0.001g���ʴ�Ϊ��ȷ������ͭ����ʧȥȫ���ᾧˮ���������γ��������������0.001g��
��4����������Ϊ22.692g-11.721g=10.971g������ͭ������Ϊ��18.631g-11.721g=6.91g��ˮ������Ϊ��22.692g-18.631g=4.061g������ͭ�����ʵ���Ϊ![]() ��ˮ�����ʵ���Ϊ
��ˮ�����ʵ���Ϊ![]() ������
������![]() ��ʵ��������Ϊ
��ʵ��������Ϊ![]() ���ʴ�Ϊ��5.22��
���ʴ�Ϊ��5.22��![]() ��
��
��5��a������ͭ�����к��в��ӷ������ʵ��½ᾧˮ����ƫС����ѡa��
b��ʵ��ǰ���������ʪ��ˮ���½ᾧˮ����ƫ��ѡb��
c������ʱ�о���ɽ���ȥ���½ᾧˮ����ƫ��ѡc��
d������ʧˮ��¶���ڿ�������ȴ����ˮ����ͭ����ˮ�����½ᾧˮ����ƫС����ѡd���ʴ�Ϊ��bc��

 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�����Ŀ��ij����С�������ͼ��ʾװ����ȡ�϶���������������֪������ˮ�Ȼ��ƿ����Ҵ��γ�������ˮ��CaCl2��6C2H5OH��
���й��л���ķе㣺
�Լ� | ���� | �Ҵ� | ���� | �������� |
�е㣨�棩 | 34.7 | 78.5 | 118 | 77.1 |
����˵����ȷ����
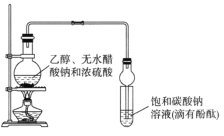
A.װ�������ιܼ��������������ܷ�ֹ����
B.��Ӧ��������Թ��е������ǣ���Һ�ֲ㣬�²���ɫ��״Һ�壻�ϲ���Һ��ɫ��dz
C.�Ӵ��Թ��з���������������л�����һ�������Ҵ������Ѻ�ˮ��Ӧ�ȼ�����ˮ�Ȼ��ƣ����˷�����Ҵ�
D.��������ˮ�����ƣ�Ȼ����������ռ�118�����ҵ���֣��Եõ��ϴ���������������