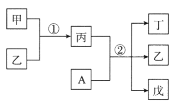
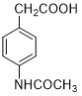
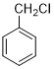
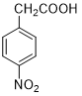
��Ŀ����
����Ŀ�����������벻����ѧ���磺����������Ƭ��������ƶѪ(��ͼ����˵����)���ڸ�������������������θ����࣬������������ˮ�����ȵȡ�

�Ķ��Ϸ�����������Ƭ���ı�ǩ˵�����ش�
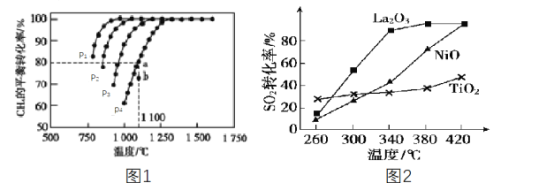
��1�� �������У�����ϡ����1�Ρ���������_____________________________________�����Ǽ�ϡ�����ԭ����_______________________________________��
��2������������������θ�����ʱ������������Ϊ_________�к���θ���������Ҳ�ܸ��ռ���Һ��Ӧ����Ӧ�����ӷ���ʽΪ_________________________��
��3�������ܾ�ˮ����Ϊ��Al3+��ˮ�⣬��ˮ�����������������____________���������һ����ʵ����֤��������ˮ������ˮ��__________________________________��
���𰸡�����FeSO4ˮ�� ��ϡ����������������ӵļ��� ������ Al(OH)3+OH��=AlO2��+2H2O ���� ��pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ�
��������
��1����������Ϊǿ�������Σ�����ˮ����ˮ�⣬�Ӷ�ʹ��Һ����ǣ���������������Ƭ�ܽ�����У�����������ˮ�⣬�ں�����֤�У�����Ҫ��֤��������ӣ��Ӽ������ữ������������������ӣ�����ԭҩƬ����������ӵļ��飬�ʴ�Ϊ������FeSO4ˮ�⣻��ϡ����������������ӵļ��飻
��2��θ��Ϊ���ᣬ�����������к��ᣬ�Ӷ����ֳ����ԣ������������ռNaOH����Һ��Ӧ�����ӷ�Ӧ����ʽΪ��Al(OH)3+OH��=
AlO2��+2H2O���ʴ�Ϊ���Al(OH)3+OH��=AlO2��+2H2O��
��3�������ܾ�ˮ����ΪAl3+��ˮ������Al(OH)3�Ľ��壬��������������������ˮ�е��������pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ����ʴ�Ϊ����������pH��ֽ��pH�Ʋ���������Һ��pH < 7 ֤����Һ�����ԣ�����ʯ����Һ����������Ҳ���ԣ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��ijͬѧ��������ͭ����(![]() )�ᾧˮ�����IJⶨʵ�顣���������գ�
)�ᾧˮ�����IJⶨʵ�顣���������գ�
��ʵ�鲽�裩��
(1)��__________(����������)ȷ������������������
(2)�ڴ������м���һ����������ͭ���壬�����ء�
(3)��ʢ������ͭ����Ĵ������������������������ȣ�ֱ����ɫ��ȫ��ף�Ȼ�����������_______(����������)����ȴ�����£������ء�
(4)�ظ�����ʵ����к��ز�������Ŀ����_______________��ֱ�����γ������������______�ˡ�
(5)�����Ǹ�ѧ��ʵ���һ�����ݣ�����ɼ��㣺
��������(��) | �����뾧�������(��) | ���غ�������������� |
13.721 | 24.692 | 20.631 |
![]() ______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
______��(��ȷ��0.01)ʵ����������_________(����С����һλ)��
(6)���ʵ���в�������ԭ�������_______
a. ����ͭ�����к��в��ӷ������� b. �ڼ��ȹ����з����к�ɫ��������
c. ����ʱ�о���ɽ����� d. ����ʧˮ��¶���ڿ�������ȴ
����Ŀ���ⶨ����ͭ���壨CuSO4XH2O ����Xֵ��ʵ��������£�
![]()
��1��������ʵ�����õ��ļ����������������Ӧ������ȷ����___________��
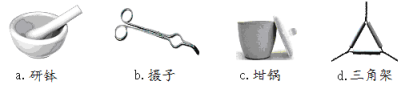
��2�������ա�ʱ��Դѡ�õ��Ǿƾ��ƶ����Ǿƾ���ƣ�������_____________________������ȴ������_______________��(����������)��
��3�� �����ء�������Ŀ����_________________________________________________��
�жϡ����ء���������_________________________________________________��
��4��������ijѧ��ʵ���һ�����ݣ�����ɼ���
�������� | �����뾧�������� | ���Ⱥ���������������� |
11.721g | 22.692g | 18.631g |
X=__________________��(��ȷ��0.01)��ʵ����������_________________��(����С�����һλ)
��5�����ʵ���в�������ԭ�������__________����ɵġ�
a������ͭ�����к��в��ӷ������ʡ��� b���ڼ��ȹ��̷����к�ɫ��������
c������ʱ�о���ɽ����������������� d������ʧˮ��¶���ڿ�������ȴ