��Ŀ����
����Ŀ��ʵ�������Ҵ���Ũ���Ṳ����ȡ��ϩ�������¶ȹ�����������SO2ij��ѧ��ȤС���������ͼ��ʾʵ�飬����֤��������������Ƿ�����ϩ��SO2�����������գ�
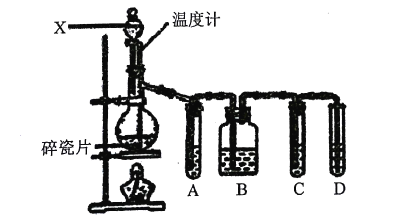
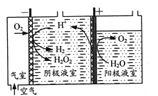
��1��װ�������Ƭ�������ǣ�___��
��2������X��������___��
��3��д��������ϩ�ķ�Ӧ����ʽ��___���÷�Ӧ�ķ�Ӧ����Ϊ��___��
��4���Թ�A��B��C��D�п�ʢ�ŵ��Լ���(�������Լ���ѡ�����Լ����ظ�ʹ�ã������)��
��Ʒ����Һ ��NaOH��Һ ����ˮ ��ϡ����
A___��B___��C___��D___��
��5��ȷ֤������ϩ��������___��
��6����û���Թ�A��B��C�������Թ�D����������Ӧ�Ļ�ѧ����ʽΪ��___��
���𰸡���ֹ���� ��Һ©�� CH3CH2OH![]() CH2=CH2��+H2O ��ȥ �� �� �� �� C����Һ����ɫ��D����Һ��ɫ CH2=CH2+Br2��CH2BrCH2Br��SO2+Br2+2H2O=H2SO4+2HBr
CH2=CH2��+H2O ��ȥ �� �� �� �� C����Һ����ɫ��D����Һ��ɫ CH2=CH2+Br2��CH2BrCH2Br��SO2+Br2+2H2O=H2SO4+2HBr
��������
��1��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170�������Ҵ��ķе�ͣ��ױ��У����Լ����Ƭ��
��2��������������״�ṹ������;���н��
��3��ʵ���������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ��
��4�����ֲ��������ʱ��Ӧ�����Ⱥ�˳��
��5��C����������D������ˮ���õ�Ϊ��ϩ��
��6����Ͷ���������ϩ���ܷ�Ӧ��
��1��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170�������Ҵ��ķе�ͣ��ױ��У����Լ����Ƭ��ֹ���У�
��2��װ����X������ͨ���������Ƶμ�Һ����ٶ������Ʒ�Ӧ�Ŀ������������������Ƿ�Һ©����
��3��ʵ���������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ���Ҵ���������ȥ��Ӧ����Ӧ����ʽΪ��CH3CH2OH![]() CH2=CH2��+H2O��
CH2=CH2��+H2O��
��4���������������Ʒ����Һ��������ϩ�ø������������Һ����ϩ�Ͷ���������ʹ�������������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�������ø������������Һ��ɫ������ϩ����װ��A��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��B�Թ�װ��NaOH��Һ��ȥSO2��װ��C�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��D ͨ����ˮ��ɫ������ϩ��
�ʴ�Ϊ���٣��ڣ��٣��ۣ�
��5����C����������D������ˮ���õ�Ϊ��ϩ������ȷ֤������ϩ��������װ��C�е�Ʒ����Һ����ɫ��D�е���ˮ��ɫ��
�ʴ�Ϊ��װ��C�е�Ʒ����Һ����ɫ��D�е���ˮ��ɫ��
��6����û���Թ�A��B��C�������Թ�D�к��еĶ���������ϩ�������巴Ӧ��ʹ����ɫ����������Ӧ�Ļ�ѧ����ʽΪ��CH2=CH2+Br2��CH2BrCH2Br��SO2+Br2+2H2O=H2SO4+2HBr��