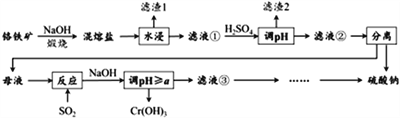
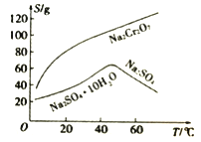
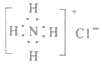��Ŀ����
����Ŀ������A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��Dͬ���壬C��Eͬ���壬D��E��Fͬ���ڣ�A��B������������֮����C��������������ȣ� A�ֱܷ���B��C�γɵ���������ȵķ��ӣ���A��C�γɵĻ����ﳣ����ΪҺ̬��A�ֱܷ���E��F�γɵ���������ȵ�������ӡ���ش��������⣺
(1)F��Ԫ�ط���Ϊ_________��E��F�ļ������а뾶�ϴ����______�������ӷ��ţ���
(2)A��C��D����Ԫ����ɵ�һ�ֳ�����������ȼҵ����Ҫ��Ʒ���û��������ʽΪ__________��
(3)B��C���⻯�����ȶ��Խ�ǿ����__________�������ʽ����
(4)B��F����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���8�����ȶ��ṹ����û�����Ļ�ѧʽΪ___________��B�ļ��⻯����F�ĵ��ʳ����¿ɷ����û���Ӧ����д���÷�Ӧ�Ļ�ѧ����ʽ��______________________��
���𰸡� Cl S2- ![]() H2O NCl3 2NH3+2Cl2=N2+6HCl
H2O NCl3 2NH3+2Cl2=N2+6HCl
��������A��B��C��D��E��F���ֶ�����Ԫ�أ����ǵ�ԭ��������������A��C�γɵĻ����ﳣ����ΪҺ̬��������Ϊˮ����AΪHԪ�أ�CΪOԪ�أ�A��B������������֮����C��������������ȣ�B������������Ϊ5����BΪNԪ�أ�A��Dͬ���壬��DΪNaԪ�أ�C��Eͬ���壬��EΪSԪ�أ�D��E��Fͬ���ڣ�A�ֱܷ���E��F�γɵ���������ȵ�������ӣ���FΪClԪ�ء�
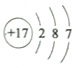
��1���������Ϸ�����FΪ��Ԫ�أ�Ԫ�ط���Cl��E��Ԫ�ط���ΪS�����Ӳ�����ͬ�����ӣ��˵����Խ�뾶ԽС���뾶S2->Cl-���뾶�ϴ����S2-��
�ʴ�Ϊ��Cl ��S2-��
��2��AΪHԪ�أ�CΪOԪ�أ�DΪNaԪ�أ���Ԫ���γɵij���������ΪNaOH���������ӻ�����������������������ӹ��ɣ�����ʽΪ![]() ��
��
�ʴ�Ϊ��![]() ��
��
��3��N�ķǽ�����С��O��NH3���ȶ��Ե���H2O���ȶ��Խ�ǿ����H2O��
�ʴ�Ϊ��H2O��
��4��BΪNԪ�أ�FΪClԪ�أ�����Ԫ���γɵ�һ�ֻ�������ӣ���ԭ�Ӿ���˵��ӽṹ���û�������NCl3��NH3��Cl2�����·����û���Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ��2NH3+2Cl2=N2+6HCl��
�ʴ�Ϊ��NCl3 ��2NH3+2Cl2=N2+6HCl��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�