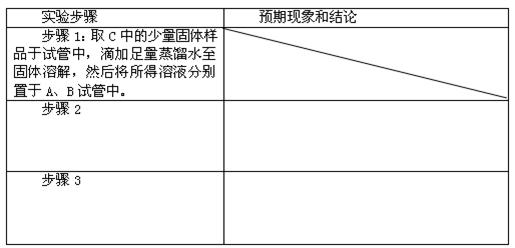
��Ŀ����
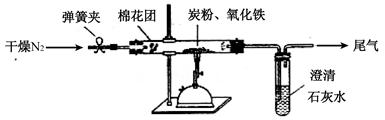
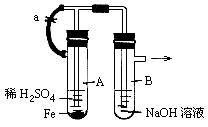
��9�֣�ijС��ͬѧΪ̽��ͭ��Ũ����ķ�Ӧ��������ռ�һƿ��Ӧ���������壬���������ͼ��ʾװ�á�ʵ��������ȡ6.4gͭƬ��12mL 18mol/LŨ�������Բ����ƿ�м��ȣ�ֱ����Ӧ����Է�����ƿ����ͭʣ�ࡣ
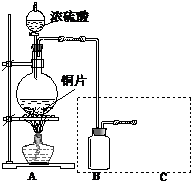
��1��Ϊ��ֹ������Ⱦ���벹����ͼ����е�ʵ��װ�ã���ע������Ҫ���Լ����ơ�
��2��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��
��3����С����ΪͭƬδ��ȫ�ܽ⣬��ƿ�п��ܻ���һ����������ʣ�࣬��ԭ���ǣ�
��
��4��Ϊ֤����Ӧ���������ƿ��ȷ�����ᣬ��ѡ������ҩƷ�е� ����д��ĸ��ţ���

��1��Ϊ��ֹ������Ⱦ���벹����ͼ����е�ʵ��װ�ã���ע������Ҫ���Լ����ơ�
��2��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��
��
��3����С����ΪͭƬδ��ȫ�ܽ⣬��ƿ�п��ܻ���һ����������ʣ�࣬��ԭ���ǣ�
��
��4��Ϊ֤����Ӧ���������ƿ��ȷ�����ᣬ��ѡ������ҩƷ�е� ����д��ĸ��ţ���
| A������ | B��BaCl2��Һ | C������ | D��Na2CO3��Һ |

��1��Ϊ��ֹ������Ⱦ��Ӧ��β������װ�ã�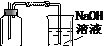
��2��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��Cu��2H2SO4(Ũ) CuSO4��SO2��+2H2O
CuSO4��SO2��+2H2O
��3����С����ΪͭƬδ��ȫ�ܽ⣬��ƿ�п��ܻ���һ����������ʣ�࣬��ԭ���ǣ�
ֻͭ��Ũ���ᷴӦ�����ŷ�Ӧ�Ľ��У�Ũ�����Ϊϡ���ᣬͭ�Ͳ��ڷ�Ӧ�����ԣ���Һ�п϶���ϡ������ڡ�
��4��Ϊ֤����Ӧ���������ƿ��ȷ�����ᣬ��ѡ�õ�ҩƷ�����ۡ�Na2CO3��Һ���������ۣ���������ܽⲢ�����ݲ�����֤�������ᡣ����Na2CO3��Һ����������ݲ�����֤�������ᡣ��ѡA D��

��2��д��ͭ��Ũ���ᷴӦ�Ļ�ѧ����ʽ��Cu��2H2SO4(Ũ)
 CuSO4��SO2��+2H2O
CuSO4��SO2��+2H2O��3����С����ΪͭƬδ��ȫ�ܽ⣬��ƿ�п��ܻ���һ����������ʣ�࣬��ԭ���ǣ�
ֻͭ��Ũ���ᷴӦ�����ŷ�Ӧ�Ľ��У�Ũ�����Ϊϡ���ᣬͭ�Ͳ��ڷ�Ӧ�����ԣ���Һ�п϶���ϡ������ڡ�
��4��Ϊ֤����Ӧ���������ƿ��ȷ�����ᣬ��ѡ�õ�ҩƷ�����ۡ�Na2CO3��Һ���������ۣ���������ܽⲢ�����ݲ�����֤�������ᡣ����Na2CO3��Һ����������ݲ�����֤�������ᡣ��ѡA D��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
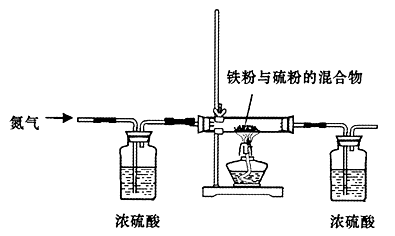
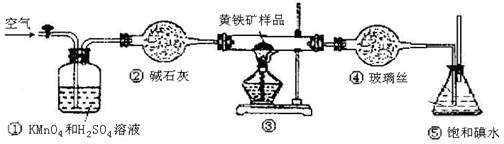

 ���Լ�Y�����ƣ�
���Լ�Y�����ƣ�