��Ŀ����
��12�֣�ij�о���ѧϰС��Թ���̿������������Ӧ���������ɷֽ����о���
(1)������裺
�ٸ÷�Ӧ�����������CO2��
�ڸ÷�Ӧ�����������CO��
�۸÷�Ӧ����������� ��
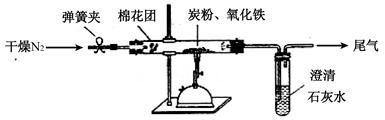
(2)�Ʒ��� ��ͼ��ʾ����һ�������������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�
(3)�������ϣ�
��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�������д���÷�Ӧ�����ӷ���ʽ�� ��
(4)ʵ�鲽�裺
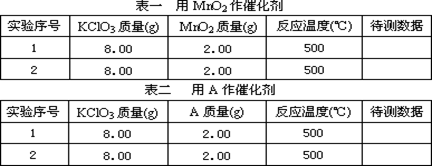
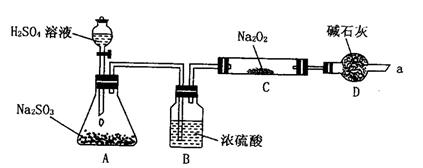
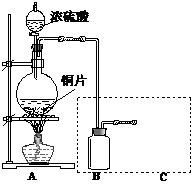
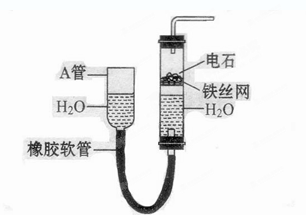
�ٰ���ͼ����װ�ã������װ�õ������ԣ���ȡ3.20g��������2.00g̼�ۻ�Ͼ��ȣ�����48.48g��Ӳ�ʲ������У�
�ڼ���ǰ����ͨһ��ʱ�䴿������ĵ�����
��ֹͣͨ��N2�н����ɼУ�����һ��ʱ�䣬����ʯ��ˮ������������ǣ�
�ܴ���Ӧ�������ٻ���ͨ��һ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24g��
�ݹ��˳�ʯ��ˮ�еij�����ϴ�ӡ���ɺ�Ƶ�����Ϊ2.00g��
����ڡ����ж��ֱ�ͨ��N2�������÷ֱ�Ϊ ��
(5)���ݴ�����
�Ը���ʵ�����ݷ�����д����ʵ������������̼������Ӧ�Ļ�ѧ����ʽ��
��
(6)ʵ���Ż���
ѧϰС����ͬѧ��ΪӦ��ʵ��װ�ý�һ�����ơ�
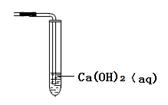
�ټ�ͬѧ��Ϊ��Ӧ������ʯ��ˮ����Ba(OH)2��Һ���������� ��
�ڴӻ��������ĽǶȣ����������һ���Ż���������ʵ��װ�ý�һ�����ƣ�
��
(1)������裺
�ٸ÷�Ӧ�����������CO2��
�ڸ÷�Ӧ�����������CO��
�۸÷�Ӧ����������� ��
(2)�Ʒ��� ��ͼ��ʾ����һ�������������ڸ��������������������̿����ȫ��Ӧ���ⶨ�μӷ�Ӧ��̼Ԫ������Ԫ�ص������ȡ�

(3)�������ϣ�
��������̼��������������Ӧ��ʵ���ҿ������Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ��ϼ��ȷ�Ӧ�Ƶõ�������д���÷�Ӧ�����ӷ���ʽ�� ��
(4)ʵ�鲽�裺
�ٰ���ͼ����װ�ã������װ�õ������ԣ���ȡ3.20g��������2.00g̼�ۻ�Ͼ��ȣ�����48.48g��Ӳ�ʲ������У�
�ڼ���ǰ����ͨһ��ʱ�䴿������ĵ�����
��ֹͣͨ��N2�н����ɼУ�����һ��ʱ�䣬����ʯ��ˮ������������ǣ�
�ܴ���Ӧ�������ٻ���ͨ��һ��ʱ��ĵ�������ȴ�����£��Ƶ�Ӳ�ʲ����ܺ���������Ϊ52.24g��
�ݹ��˳�ʯ��ˮ�еij�����ϴ�ӡ���ɺ�Ƶ�����Ϊ2.00g��
����ڡ����ж��ֱ�ͨ��N2�������÷ֱ�Ϊ ��
(5)���ݴ�����
�Ը���ʵ�����ݷ�����д����ʵ������������̼������Ӧ�Ļ�ѧ����ʽ��
��
(6)ʵ���Ż���
ѧϰС����ͬѧ��ΪӦ��ʵ��װ�ý�һ�����ơ�
�ټ�ͬѧ��Ϊ��Ӧ������ʯ��ˮ����Ba(OH)2��Һ���������� ��
�ڴӻ��������ĽǶȣ����������һ���Ż���������ʵ��װ�ý�һ�����ƣ�
��
��CO2��CO�Ļ����
��NH4++NO2�� N2��+2H2O
N2��+2H2O
�Ȳ��������Ϊ���ų������е�CO2���������Ϊ�˸ϳ����е�CO2��ȷ����ȫ����
��2C+Fe2O3 2Fe+CO��+CO2��
2Fe+CO��+CO2��
�ʢ�Ba(OH)2�ܽ�ȴ�Ũ�ȴ�ʹCO2�����յĸ���ȫ��M (BaCO3)��M(CaCO3)������ʱ������С������β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
��NH4++NO2��
 N2��+2H2O
N2��+2H2O�Ȳ��������Ϊ���ų������е�CO2���������Ϊ�˸ϳ����е�CO2��ȷ����ȫ����
��2C+Fe2O3
 2Fe+CO��+CO2��
2Fe+CO��+CO2�� �ʢ�Ba(OH)2�ܽ�ȴ�Ũ�ȴ�ʹCO2�����յĸ���ȫ��M (BaCO3)��M(CaCO3)������ʱ������С������β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
�����������1�����ݼ��˼����֪����һ������������ߵĻ�����CO2��CO�Ļ���
��3�����Ȼ�隣�����Һ���������ƣ�NaNO2��������Һ�Ƶ�����Ӧ�����ӷ���ʽΪ��NH4++NO2��
 N2��+2H2O��
N2��+2H2O����4�������ͨ�뵪����Ŀ�����ų�װ���е�CO2�������ͨ�뵪����Ŀ���ǽ����ɵ�CO2ȫ��ͨ�����ʯ��ˮ�С�
��5���������֪̼�۹�����������CO2Ϊ0.02mol��ʣ��Ĺ���Ϊ3.76g�����Թ������е�����2.24g����ʣ���̼��Ϊ3.76-2.24=1.52g�����Բμӷ�Ӧ��̼��Ϊ2-1.52=0.48g=0.04mol�������ȷ������COΪ0.02mol����˷���ʽΪ��2C+Fe2O3
 2Fe+CO��+CO2����
2Fe+CO��+CO2������6����Ӧ������ʯ��ˮ����Ba(OH)2��Һ��ԭ����Ba(OH)2�ܽ�ȴ�Ũ�ȴ�ʹCO2�����յĸ���ȫ��M (BaCO3)��M(CaCO3)������ʱ������С���ڴӻ����ĽǶ��������ɵ�COҲҪ�����������Ӧ����β�����ڴ���һ��ȼ�ľƾ��ƻ�����һβ������װ��
�����������ۺ��Խ�ǿ���ѶȽϴ���Ҫ����ѧ����������ͽ�������������

��ϰ��ϵ�д�
�����Ŀ
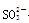 ��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�
��Cl�� ��Br�����е������֣����ν�������ʵ�顣�۲쵽�������£�