��Ŀ����
����Ŀ��������й���ѧԺ����������CO2��ת��ΪCO���о�����³ɹ�����ͼ��ʹ�ò�ͬ����(NiPc��CoPc)ʱת�������е������仯������˵����ȷ����
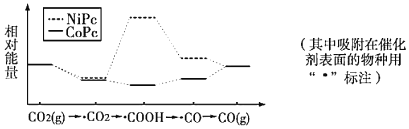
A.CO2(g)��CO(g)+l/2O2(g)��������������
B.CO2��������Ӧ�õ�COOH
C.�������£�����NiPc��CoPc��CO2(g)��CO(g)ת��Ч�ʸ���
D.���о��ɹ��������ڻ�������ЧӦ�������Դת������
���𰸡�D
��������
A��CO2(g)��CO(g)+l/2O2(g)�����ȷ�Ӧ���������������ߣ�Aѡ�����
B��CO2�õ�COOH�Ĺ��̣�C�Ļ��ϼ���+4�۽�����+3�ۣ�������ԭ��Ӧ��Bѡ�����
C���������£�����NiPc��CoPc��CO2(g)��CO(g)ת��Ч�ʸ��ͣ�Cѡ�����
D�����о��ɹ���CO2����COOH��CO��CO���п�ȼ�ԣ����������ڻ�������ЧӦ�����ܽ����Դת�����⣬Dѡ����ȷ��
��ѡD��

��ϰ��ϵ�д�
 ͬ����ϰ���ϴ�ѧ������ϵ�д�
ͬ����ϰ���ϴ�ѧ������ϵ�д� ͬ����ϰ����ʦ����ѧ������ϵ�д�
ͬ����ϰ����ʦ����ѧ������ϵ�д�
�����Ŀ