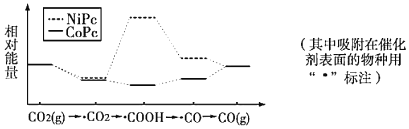
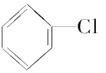
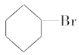
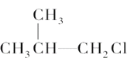��Ŀ����
����Ŀ���ڳ����£�Fe��ˮ������Ӧ�����ڸ����£�Fe��ˮ�����ɷ�����Ӧ��Ӧ������װ�ã���Ӳ�ʲ������з��뻹ԭ���ۺ�ʯ���Ļ������ȣ���ͨ��ˮ�������Ϳ�����ɸ�������Fe��ˮ�����ķ�Ӧʵ������ ��ش��ʵ���е����⡣
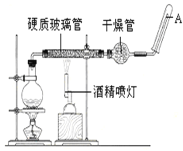
��1��д���÷�Ӧ�Ļ�ѧ����ʽ____________________��Բ����ƿ��ʢװˮ����ƿ��Ӧ���ȷ���____����������_____��
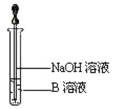
��2����ͬѧ��ȷ����Ӧ��Ӳ���Թ��й������ʵijɷ֣����������ʵ�鷽�����ٴ�Ӳ���Թ���ȴ��ȡ�������еĹ�����������ϡ�������ҺB����ȡ������ҺB�μ�KSCN��Һ����Һδ���ɫ,��ͬѧ������ȡ������ҺB��ʹ���NaOH��Һ��Ӧ������ͼ��ʾ�IJ������ɹ۲쵽________________________________________ ��������д��������������صķ�Ӧ�Ļ�ѧ����ʽ___________________________ ��____________________________________��
���𰸡�3Fe+4H2O(g) ![]() Fe3O4+4H2 ���Ƭ ��ֹ���� �����ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ FeSO4+2NaOH=Fe(OH)2+Na2SO4 4Fe(OH)2+O2+2H2O= 4Fe(OH)3
Fe3O4+4H2 ���Ƭ ��ֹ���� �����ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ FeSO4+2NaOH=Fe(OH)2+Na2SO4 4Fe(OH)2+O2+2H2O= 4Fe(OH)3
��������
��1����������ˮ������Ӧ�Ļ�ѧ����ʽ���̲����ѳ��֣�������д����ѧ����ʽ����ԭ�����ж��������ݻ��ϼ����ߵ����ʣ��������ǻ��ϼ۽��͵����ʣ�����3Fe+4H2O(g) ![]() Fe3O4+4H2 �� ��Ӧ��Ϊˮ���������������Ƭ��Ŀ���Ƿ�ֹ�����¹ʷ�����
Fe3O4+4H2 �� ��Ӧ��Ϊˮ���������������Ƭ��Ŀ���Ƿ�ֹ�����¹ʷ�����
��2������NaOH��Һʱ�����ɵİ�ɫ������������������������������������Ϊ�������������ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��
��1������ˮ��������������ԭ��Ӧ��3Fe+4H2O(g) ![]() Fe3O4+4H2����Ϊ��Ӧ��Ϊˮ��������������������ȵ�Ŀ�ľ����ṩˮ��������ƿ�ײ�Ӧ���ȷ������Ƭ�������Ƭ��Ŀ���Ƿ�ֹ�����¹ʷ�����
Fe3O4+4H2����Ϊ��Ӧ��Ϊˮ��������������������ȵ�Ŀ�ľ����ṩˮ��������ƿ�ײ�Ӧ���ȷ������Ƭ�������Ƭ��Ŀ���Ƿ�ֹ�����¹ʷ�����
�ʴ�Ϊ��3Fe+4H2O(g) ![]() Fe3O4+4H2�����Ƭ����ֹ���У�
Fe3O4+4H2�����Ƭ����ֹ���У�
��2������NaOH��Һʱ�����ɵİ�ɫ������������������������������������Ϊ�������������ɫ����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ�������ķ�ӦΪFeSO4+2NaOH=Fe��OH��2��+Na2SO4��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ�������ɰ�ɫ������Ѹ�ٱ�Ϊ����ɫ������Ϊ���ɫ��FeSO4+2NaOH=Fe��OH��2��+Na2SO4��4Fe��OH��2+O2+2H2O=4Fe��OH��3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����Ԫ�غ�±��Ԫ�ض����γɶ������ʣ����ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ�����⡣
��1��COCl2�Ŀռ乹��Ϊ______________����Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ_____________��
��2����֪CsICl2���ȶ��������ֽ⣬���������ɾ����ܸ�������ʣ�����������_____________������ĸ��ʽ������
A.CsICl2====CsCl+ICl B.CsICl2====CsI+Cl2
��3�����ݱ��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����____��
Ԫ�� | �� | �� | �� | �� |
��һ������/ ��kJ��mol-1�� | 1681 | 1251 | 1140 | 1008 |
��4�����з��ӼȲ����ڡ�s-p���Ҽ���Ҳ�����ڡ�p-p���м�����__________������ĸ����
A.HCl B.HF C.SO2 D.SCl2
��5����֪ClO2��Ϊ��V���Σ�������ԭ����Χ��4�Լ۲���ӡ�ClO2��������ԭ�ӵ��ӻ��������Ϊ______________��д��һ����CN����Ϊ�ȵ���������ʵķ���ʽ��______________��
��6������������ȼ��ʱ�õ�һ�ָƵ������ᄃ�壬��ṹ��ͼ��ʾ���ɴ˿��жϸƵ�������Ļ�ѧʽΪ__________����֪����������ܶ��Ǧ�g��cm-3�����������������������ӵļ��Ϊ_________cm��ֻҪ������ʽ�����ؼ������ֵ�������ӵ�������ֵΪNA����