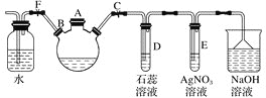
��Ŀ����
����Ŀ����һ�ݻ�Ϊ2 L���ܱ������ڼ���0.2 mol N2��0.6 mol H2����һ�������·������·�Ӧ��N2(g)��3H2(g)![]() 2NH3 ��H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ��
2NH3 ��H��0����Ӧ��NH3�����ʵ���Ũ�ȵı仯�����ͼ��ʾ��
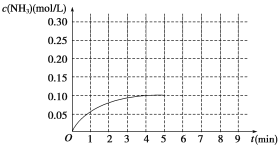
��1������ͼʾ������ӷ�Ӧ��ʼ����4���Ӵﵽƽ��ʱ��ƽ����Ӧ���ʦ�(N2)Ϊ__________��
��2���ﵽƽ���5����ĩ�������������������䣬ֻ�ı䷴Ӧ�¶ȣ���NH3�����ʵ���Ũ�Ȳ�����Ϊ_____________��
a��0.20 mol/L b��0.12 mol/L c��0.10 mol/L d��0.08 mol/L
��3���ﵽƽ���5����ĩ�������������������䣬ֻ�������������С����ƽ��ʱNH3��Ũ��ǡ��Ϊԭ����2�����������_____(ѡ����ڡ��������ڡ�����С�ڡ�)����֮һ����ԭ�������ѧƽ�ⳣ��________(ѡ���������С�����䡱)��
��4���ڵ�5����ĩ�������������Сһ�룬���ڵ�8����ĩ�ﵽ�µ�ƽ��(��ʱNH3��Ũ��ԼΪ0.25 mol/L)������ͼ�л�����5����ĩ������ƽ��ʱNH3Ũ�ȵı仯����_____��
��5����֪��1mol H��H����1molN��H����1molN��N���ֱ���Ҫ��������436kJ��391kJ��946kJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ________________________________________________
���𰸡�0.0125mol��L��1��min��1ac���ڲ���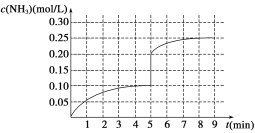 N2(g)+3H2(g)
N2(g)+3H2(g)![]() 2NH3(g) ��H=-92KJ/mol
2NH3(g) ��H=-92KJ/mol
��������
��1������ͼʾ������ӷ�Ӧ��ʼ����4���Ӵﵽƽ��ʱ��c(NH3)=0.10mol/L����(NH3)=0.10/4=0.025 mol��L��1��min��1,��������֮�Ⱥ�ϵ�������ȹ�ϵ��֪����(N2)=1/2��(NH3)= 0.025/2=0.0125mol��L��1��min��1����˱��������0.0125mol��L��1��min��1��
(2)�÷�ӦΪ���淴Ӧ,��Ӧ�ﲻ������ȫת��,�� NH3�����ʵ���Ũ�Ȳ�����Ϊ0.20 mol/L ,�¶ȸı�ƽ��һ�������ƶ����� NH3�����ʵ���Ũ�Ȳ�����Ϊ0.10 mol/L ����ˣ�������ȷ����: ac��
��3���ﵽƽ���5����ĩ�������������������䣬ֻ�������������СΪԭ����2�������û��ƽ���ƶ���NH3��Ũ��ǡ��Ϊԭ����2�������Ǹ÷�ӦΪ���淴Ӧ������ѹǿƽ�����ƣ�NH3��Ũ�ȴ���ԭ����2����Ҫ�����ԭ����2��������������ڶ���֮һ����ԭ�����ƽ�ⳣ��ֻ���¶��йأ���С����Ĺ��̣��¶�û�з����仯�� ��˻�ѧƽ�ⳣ������������������������������ڣ� ���䡣
��4���ڵ�5����ĩ�������������Сһ�룬������Ũ�ȱ�Ϊԭ����һ����ѹǿ����ƽ�����������ƶ���������Ũ��������,��8����ʱ�ﵽƽ����Ũ��ԼΪ0.25 mol/L����5����ĩ������ƽ��ʱNH3Ũ�ȵı仯����������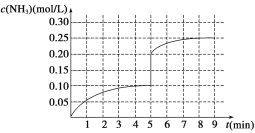 ������������������ǣ�
������������������ǣ� ��
��
��5���ڷ�ӦN2(g)��3H2(g)![]() 2NH3 ��������3mol H��H��, 1molN��N�������յ�����Ϊ:3��436+946=2254kJ,����2molNH3,���γ�6molN��H�����ų�������Ϊ��6��391=2346 kJ,���յ�������,�ų���������,�÷�ӦΪ���ȷ�Ӧ,�ų�������Ϊ:2346-2254=92kJ����ˣ�������ȷ����N2(g)+3H2(g)
2NH3 ��������3mol H��H��, 1molN��N�������յ�����Ϊ:3��436+946=2254kJ,����2molNH3,���γ�6molN��H�����ų�������Ϊ��6��391=2346 kJ,���յ�������,�ų���������,�÷�ӦΪ���ȷ�Ӧ,�ų�������Ϊ:2346-2254=92kJ����ˣ�������ȷ����N2(g)+3H2(g)![]() 2NH3(g) ��H=-92KJ/mol��
2NH3(g) ��H=-92KJ/mol��

 ����ʦ���һ��һ��ϵ�д�
����ʦ���һ��һ��ϵ�д�����Ŀ����1����K2Cr2O7��ˮ��Һ�м���Ba��NO3��2��Һ,���������Ի�ɫ����BaCrO4��˵��K2Cr2O7��ˮ��Һ�д�������ƽ�⣺__________________________________________,������������ҺPH��________.��֪����ˮ��Һ��K2Cr2O7Ϊ�Ⱥ�ɫ��K2CrO4Ϊ��ɫ����������Һ�м�������������Һ��_________ɫ�����Ѽ����������Ƶ���Һ�У��ټ������������Һ��___________ɫ����ʵ����������������ԭ��������ı�ά�ֻ�ѧƽ���������Ũ�ȡ�ѹ�����¶ȣ���ƽ��ͻ�����_________���ָı�ķ����ƶ���
��2����KMnO4��H2C2O4��Ӧ�У���ͨ���ⶨ_________________���ⶨ�÷�Ӧ�����ʣ�д������������KMnO4��H2C2O4�����ᣩ��Ӧ�����ӷ�Ӧ����ʽ��____________________________���˷�Ӧ��ʼ��Ӧ���������ӦѸ�ټӿ죬��ԭ����______������ĸ��
A��KMnO4��Һ�е�H+������� B�����ɵ�CO2�ݳ���ʹ������Ũ�Ƚ���
C����Ӧ�У����ɵ�Mn2+������� D��������������KMnO4����������ǿ
��3��Ϊ̽�ֻ�ѧ��Ӧ���ʵ�Ӱ�����أ���Ƶ�ʵ�鷽�����±�������֪ I2��2S2O32-===S4O62-��2I��������Na2S2O3��Һ��������
ʵ�� ��� | ���V/mL | ʱ��/s | |||
Na2S2O3��Һ | ������Һ | ��ˮ | ˮ | ||
�� | 10.0 | 2.0 | 4.0 | 0.0 | t1 |
�� | 8.0 | 2.0 | 4.0 | 2.0 | t2 |
�� | 6.0 | <>2.0 | 4.0 | Vx | t3 |
�ٸ�ʵ����е�Ŀ����_____________������Һ��������____________��
�ڱ���Vx=_______mL���Ƚ�t1��t2��t3��С____________�����Ʋ��ʵ����ۣ� ____________
����Ŀ��CO��;�㷺����ҵӦ��ʱ�벻��ƽ��˼���ָ����
��.��ijһ�ݻ�Ϊ5L�����������ܱ������ڣ�����0.3 mol ��CO��0.3mol��H 2O���ڴ������ں�800��������¼��ȣ��������·�Ӧ��CO��g��+H20��g��CO2��g��+H2��g�� ��H��0����Ӧ��CO2��Ũ����ʱ��仯�������ͼ��
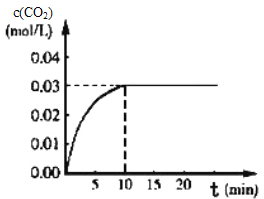
��1������ͼ�����ݣ����¶ȣ�800�棩�µ�ƽ�ⳣ��K=____________________________��
��2�����������������£��ı�����������ʹƽ�ⳣ��K�������______________������ĸ��
A.�����¶� B.�����¶�
C.����ѹǿ D.��Сѹǿ
E.������� G.�Ƴ�һ����̼����
��3���������¶Ⱥ�������������䣬�ڣ�1��������ƽ����ϵ�У��ٳ���0.3mol��ˮ���������´ﵽƽ���H2O��ת����______________������ߡ��������͡��������䡱����
��4���ڴ������ں�800��������£���ijһʱ�̲��c��CO��=c��H2O��=0.09mol/L��c��CO2��=c��H2��= 0.13mol/L�����ʱ�����淴Ӧ���ʵĴ�С������______________�������>������<����=������
��ԭ����Ǧ��������ӦPbO��s��+CO��g��Pb��s��+C02��g�� ��H���÷�Ӧ��ƽ�ⳣ���Ķ���ֵ���¶ȵĹ�ϵ���±���
�¶�/�� | 300 | 727 | 1227 |
lgK | 6.17 | 2.87 | 1.24 |
��5���÷�Ӧ�ġ�H______________0��ѡ�>������������=������
��6����lgK=1���ں����ܱ������з���PbO��ͨ��CO����ƽ��ʱ�����������CO���������Ϊ____��������λ��Ч���֣������������г���һ������CO�����ƽ�ⷢ���ƶ����ٴδﵽƽ��ʱ��CO�İٷֺ���______________�����������С�����䡱����