��Ŀ����
16����1��MgCl2��ˮ��Һ���ᣨ��ᡱ�����С���������ԣ�ԭ���ǣ������ӷ���ʽ��ʾ����Mg2++2H2O?Mg��OH��2+2H+��2��Ϊ�˳�ȥMgCl2������Һ�е�Fe3+�����ڼ��Ƚ���������¼���һ���Լ������˺�������Һ�м������������ᣬ�����Լ���B
A��NaOHB��MgO C��Na2CO3 D��NH3•H2O
��3����25���£���a mol•L-1�İ�ˮ��0.01mol•L-1������������ϣ���÷�Ӧ�����Һ��c��NH4+��=c��Cl-��������Һ���� �ԣ���ᡱ��������С�����
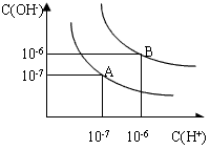
��4������A���ʾ25��ʱˮ�ڵ���ƽ��ʱ������Ũ�ȣ����¶����ߵ�100��ʱ��ˮ�ĵ���ƽ��״̬�ﵽB�㣬���ʱˮ�����ӻ���10-12����pH=8��Ba��OH��2��Һ��pH=5��ϡ�����ϣ�����100��ĺ��£���ʹ�����ҺpH=7����Ba��OH��2����������֮��Ϊ10��1��
��5��25��ʱ����Ũ�Ⱦ�Ϊ0.1mol•L-1��MgCl2��CuCl2�����Һ����μ��백ˮ��������Cu��OH��2�������ѧʽ�������ɸó��������ӷ���ʽΪCu2++2NH3•H2O=Cu��OH��2��+2NH4+������֪25��ʱKsp[Mg��OH��2]=1.8��10-11��KsP[Cu��OH��2]=2.2��10-20��
���� ��1��MgCl2��ˮ��Һ��þ����ˮ�⣬��Һ�����ԣ�
��2��Fe3+��ˮ�����ɳ�������ͨ��������ҺpH�ķ����ٽ�Fe3+��ˮ�⣬ע�ⲻ�������µ����ʣ�
��3����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.005mol/L�����������غ��n��NH3��H2O��=��0.5a-0.005��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ�
��4�����ͼʾ����ˮ�����ӻ�����ʽKw=c��H+��•c��OH-��������������Ũ�ȼ����25��ʱ��100��ˮ�����ӻ����������������Һ��������Һ�������Ȼ�������Һ��pH��ʽ��������ߵ�����ȣ�
��5�����ܵ���ʵ��ܶȻ�ԽС�����백ˮʱԽ�����ɳ�����
��� �⣺��1��MgCl2��ˮ��Һ��þ����ˮ�⣬��Һ�����ԣ���Ӧ�����ӷ���ʽΪ��Mg2++2H2O?Mg��OH��2+2H+���ʴ�Ϊ���Mg2++2H2O?Mg��OH��2+2H+��
��2��FeCl3��ˮ��������������������Mg��OH��2��ĩ��MgO��MgCO3��������Һ��pH���ٽ������ӵ�ˮ�⣬�Ҳ������µ����ʣ��ʴ�Ϊ��B��
��3����25���£�ƽ��ʱ��Һ��c��NH4+��=c��Cl-��=0.005mol/L�����������غ��c��NH3��H2O��=��0.5a-0.005��mol/L�����ݵ���غ��c��H+��=c��OH-��=10-7mol/L����Һ�����ԣ��ʴ�Ϊ���У�
��4��A��25��ʱ��c��H+��=c��OH-��=1��10-7mol/L��Kw=c��H+��•c��OH-��=1��10-7��1��10-7=10-14��
100��ʱ��c��H+��=c��OH-��=1��10-6mol/L��Kw=c��H+��•c��OH-��=1��10-6��1��10-6=10-12��
��������������Һ�����ΪxL����������Ϊy��pH=8��Ba��OH��2��Һ����Һ��c��OH-��=1��10-6mol/L��
pH=5��ϡ������Һ��c��H+��=1��10-5mol/L��
��ʹ�����ҺpH=7����������n��H+��=n��OH-������1��10-6mol/L��xL=1��10-5mol/L��yL��
���x��y=10��1��
�ʴ�Ϊ��10-12��10��1��
��5�����ܵ���ʵ��ܶȻ�ԽС�����백ˮʱԽ�����ɳ����������ɵij���ΪCu��OH��2����Ӧ�����ӷ���ʽΪCu2++2NH3•H2O=Cu��OH��2��+2NH4+��
�ʴ�Ϊ��Cu��OH��2��Cu2++2NH3•H2O=Cu��OH��2��+2NH4+��
���� ���⿼����ˮ�ĵ��뼰ˮ�����ӻ��ļ��㣬ע���˻���֪ʶ�Ŀ��飬ע�ⴿˮ��c��H+��=c��OH-���������ѶȲ���

 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�| A�� | 7.2 g | B�� | 4.2 g | C�� | 3.6 g | D�� | 2.1 g |
| ���� | �� | �� | �� |
| ��Ӧ��Ͷ���� | 1mol N2��3mol H2 | 2mol NH3 | 4mol NH3 |
| NH3��Ũ�ȣ�mol•L-1�� | c1 | c2 | c3 |
| ��Ӧ�������仯 | �ų�a kJ | ����b kJ | ����c kJ |
| ��ϵѹǿ��Pa�� | p1 | p2 | p3 |
| ��Ӧ��ת���� | ��1 | ��2 | ��3 |
| A�� | 2c1��c3 | B�� | a+b=92.4 | C�� | 2p2��p3 | D�� | ��1+��2��1 |
| A�� | Na+��Fe2+��Cl-��SO42- | B�� | K+��Ag+��Cl-��NO3- | ||
| C�� | Ba2+��Na+��NO3-��CO32- | D�� | Na+��K+��CO32-��SO32- |
| A�� | �õ�ط�ӦΪ���淴Ӧ | |
| B�� | �ŵ�ʱ��Li+���ƶ� | |
| C�� | ���ʱ��������ӦʽΪLi++e-�TLi | |
| D�� | �õ�صĵ������Һ���Ի���LiBr��ˮ��Һ |
| A�� | ʳ�� | B�� | ���� | C�� | ���� | D�� | �� |
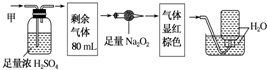
| A�� | һ����NH3�������Ϊ20mL | B�� | һ����CO2�������Ϊ48mL | ||
| C�� | һ����NO�������Ϊ30mL | D�� | ��������һ������NO2��N2��O2 |
| A�� | ��ˮ����ȡ�嵥�ʿ�������������±�е�Br-���� | |
| B�� | þ���Ż���ö�����̼��� | |
| C�� | Һ���ʢ���ڴ������Ĺ��ƿ�� | |
| D�� | ��ˮ����ȡþ���ʵķ����ǣ���ˮ��Mg��OH��2��Mg |
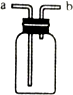 ��ͼװ������ѧ��ѧʵ���г�������������������ϴ���⣬����������;��
��ͼװ������ѧ��ѧʵ���г�������������������ϴ���⣬����������;��