��Ŀ����
����Ŀ����ͬѧ��Ʋ�����������ʵ�飬��̽����Ȳ����ļӳɷ�Ӧ����ȡһ������ҵ�õ�ʯ��ˮ��Ӧ�������ɵ�����ͨ����������ˮ�У�������Һ��ɫ����֤����Ȳ����ˮ�����˼ӳɷ�Ӧ����ͬѧ�����ڼ�ͬѧ��ʵ���У���ɫ����Һ����������ɫ���ǣ��Ʋ����Ƶ���Ȳ�л����ܺ���������ԭ�Ե��������壬�ɴ�����������ȳ�ȥ֮��������ˮ��Ӧ������ش��������⣺
��1��д����ͬѧʵ���е�������ѧ����ʽ��_______________��____________��
��2������Ϊ����Ƶ�ʵ�鲻����֤�˷�ӦΪ�ӳɷ�Ӧ����������________________��
A��ʹ��ˮ��ɫ�����ʣ�������Ȳ
B��ʹ��ˮ��ɫ�ķ�Ӧ���Ǽӳɷ�Ӧ
C��ʹ��ˮ��ɫ������,δ������Ȳ
D��ʹ��ˮ��ɫ�ķ�Ӧδ���Ǽӳɷ�Ӧ
��3����ͬѧ�Ʋ����Ȳ�бض����е�һ������������_________,����֤�����б���ȫ����ȥ��������ˮ��Ӧ�Ļ�ѧ����ʽ��________________________________________________________��
��4������ѡ����������װ��(����ͼ)�����ظ�ʹ�ã���ʵ����ͬѧ��ʵ�鷽���������ǵı�����뷽��д��װ�������ŵĻ�ѧҩƷ��
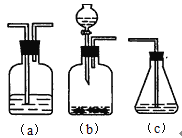
![]()
___��___��___��___
��5��Ϊ��֤��һ��Ӧ�Ǽӳɶ�����ȡ������ͬѧ�������pH��ֽ�����Է�Ӧ����Һ�����ԣ�������___��
���𰸡�CaC2+2H2O��Ca(OH)2+C2H2�� CH��CH+2Br2��CHBr2��CHBr2 C ��D H2S Br2+H2S��S��+2HBr b a CuSO4��Һ CuSO4��Һ ��������ȡ����Ӧ���ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤
��������
��1����ʯ��ˮ��Ӧ������Ȳ����Ӧ�ķ���ʽΪCaC2+2H2O��Ca(OH)2+C2H2������Ȳ����̼̼������������ˮ�����ӳɷ�Ӧ��ʹ��ˮ��ɫ����Ӧ�Ļ�ѧ����ʽΪCH��CH+2Br2��CHBr2��CHBr2��
��2��A.��ʹ��ˮ��ɫ�����ʲ�һ��������Ȳ��A����B.ʹ��ˮ��ɫ�ķ�ӦҲ��һ�����Ǽӳɷ�Ӧ��B����C.ʹ��ˮ��ɫ������δ������Ȳ��C��ȷ��D.ʹ��ˮ��ɫ�ķ�ӦҲδ���Ǽӳɷ�Ӧ��D��ȷ����ѡCD��
��3����ʯ�к������ʣ���ˮ���ɵ���Ȳ�к���H2S��������л�ԭ�ԣ��ܰ���ˮ��������Ӧ�Ļ�ѧ����ʽΪBr2+H2S��S��+2HBr��
��4��������������ͭ��Һ��Ӧ���ɺ�ɫ������ͭ��������Ҫ��������ͭ��ȥ���⡣Ϊ�����Ƿ����������Ҫ�ٴ�ͨ������ͭ��Һ��
��5�������������ȡ����Ӧ����ض�����HBr����Һ���Խ���������ǿ���ʿ���pH��ֽ��֤��

����Ŀ��п������п�����̵��ң���ZnO 35%���ϣ�������������������(MnO)������ͭ�� ����������Ͳ�����������ʣ���ҵ�ϳ������������ ZnSO4��7H2O����֪ ZnSO4��7H2O ����������ˮ�������ھƾ���ij��ȤС��ʵ����ģ����� ZnSO4��7H2O ���壬�������£�

��ش�
(1)�������� II �еIJ�����ԭ���� �ش��������⣺
�ٽ�ϱ� 1�� 2������ѡ��� pH ���¶ȷֱ���______________________�� ���У� ���Բ��ü���_________________________������ pH ��
��1 pH��ZnSO4.7H2O �����������ȵ�Ӱ��
pH | ZnSO4.7H2O ��������g�� | ��Ʒ��Fe�ĺ���% | ��Ʒ�������ؽ�������% |
1 | 114.32 | 0.750 | 0.059 |
2 | 114.4 | 0.086 | 0.056 |
3 | 113.68 | 0.034 | 0.054 |
4 | 113.60 | 0.010 | 0.050 |
5 | 112.43 | 0.010 | 0.050 |
��2 �¶ȶ�ZnSO4.7H2O �����������ȵ�Ӱ��
�¶� (��) | ZnSO4.7H2O ��������g�� | ��Ʒ��F�ĺ���% | ��Ʒ�������ؽ�������% |
20 | 111.45 | 0.011 | 0.052 |
40 | 112.89 | 0.010 | 0.051 |
60 | 113.30 | 0.010 | 0.050 |
80 | 113.80 | 0.010 | 0.050 |
90 | 114.40 | 0.091 | 0.048 |
������ KMnO4 ��Һ����Һ�е� Fe2+�������������ֳ�����ͬʱ�������ĸ�����������Ե��������Զ��ֽ�����MnO2 ��������д���ڸû����£�KMnO4 ��Һ���� Fe2+�����ӷ�Ӧ����ʽ_________________________________________�� ����ϡ�����������������˲����п��ܺ��� Zn(NO3)2 �⣬�����ܵ�ȱ���ǣ�_________________________��
(2)��������ʵ������У��ش��������⣺
������ B ����Ҫ�ɷ�Ϊ___________________________��
����μ�����ҺB���Ƿ�����Ԫ��_____________________________________��
��д����������C�����ӷ���ʽ__________________________________________��
(3)Ϊ�ⶨ ZnSO4��7H2O ����Ĵ��ȣ����� K4Fe(CN)6 ��Һ���еζ�����Ҫԭ�����£�2K4Fe(CN)6+ 3ZnSO4= K2Zn3[Fe(CN)6]2��+ 3K2SO4
ȷ��ȡ 5.000g ZnSO4��7H2O ���壬������ˮ�ܽⲢ������ 250mL��ȷ��ȡ����Һ 25.00mL����ƿ�У��� 0.0500mol/L K4Fe(CN)6 ��Һ���еζ��������������±���
ʵ����� | �ζ�ǰ����/mL | �ζ������/mL |
1 | 0.10 | 19.92 |
2 | 1.34 | 21.12 |
3 | 0.00 | 20.10 |
�� ZnSO4��7H2O ����Ĵ�����_______________(������������ʾ��������С�������λ)��