��Ŀ����
����Ŀ�������Ȼ�ѧ����ʽ���й�Ӧ�õ������У���ȷ����
A.�����ȼ����Ϊ890.3kJmol-1�������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪ�� CH4(g)+![]() O2(g) �T CO(g) + 2H2O(l) ��H = -890.3kJmol-1
O2(g) �T CO(g) + 2H2O(l) ��H = -890.3kJmol-1
B.��֪ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l) ��H����57.3kJ��mol��1����ϡ������ϡ����������Һ��Ӧ����1molˮʱ�ų�57.3kJ������
C.500���£���0.5mol I2(g)��0.5molH2(g)�����ܱյ������г�ַ�Ӧ����HI(g)������10kJ�����Ȼ�ѧ����ʽΪ��I2(g) + H2(g)![]() 2HI(g) ��H = -20kJmol-1
2HI(g) ��H = -20kJmol-1
D.��֪25����101KPa�����£�4Al(s) + 3O2(g) �T 2A12O3(s) ��H = -2834.9kJmol-1�� 4Al(s) + 2O3(g) �T 2A12O3(s) ��H = -3119.1kJmol-1����O2��O3�ȶ�
���𰸡�D
��������
A�������ȼ������ָ1mol������ȫȼ�ղ�����Һ̬ˮ���ͷŵ������������ȼ�յ��Ȼ�ѧ����ʽ�ɱ�ʾΪCH4(g)+2O2(g)�TCO2(g)+2H2O(l)��H=-890.3KJmol-1����A����
B��ϡ��Һ�У�H��(aq)��OH��(aq)===H2O(l) ��H����57.3kJ��mol��1�����������ᣬ����ʱ������������ϡ������ϡ����������Һ��Ӧ����1molˮʱ�ų�������С��57.3kJ����B����
C��I2(g) + H2(g)![]() 2HI(g)Ϊ���淴Ӧ��0.5mol I2(g)��0.5molH2(g)�����ܱյ������г�ַ�Ӧ����HI(g)������10kJ����1mol I2(g) ��ȫ��Ӧʱ���ȴ���20kJ���Ȼ�ѧ����ʽΪ��I2(g) + H2(g)
2HI(g)Ϊ���淴Ӧ��0.5mol I2(g)��0.5molH2(g)�����ܱյ������г�ַ�Ӧ����HI(g)������10kJ����1mol I2(g) ��ȫ��Ӧʱ���ȴ���20kJ���Ȼ�ѧ����ʽΪ��I2(g) + H2(g)![]() 2HI(g) ��H ��-20kJmol-1����C����
2HI(g) ��H ��-20kJmol-1����C����
D���ɢ�4Al(s)+3O2(g)�T2A12O3(s)��H=-2834.9KJmol-1����4Al(s)+2O3(g)�T2A12O3(s)��H=-3119.1KJmol-1����ϸ�˹���ɿ�֪����-�ڵõ�3O2(g)�T2O3(g)��H=-2834.9KJmol-1-(-3119.1KJmol-1)��0����֪�����������ͣ���O2��O3�ȶ�����D��ȷ��
�ʴ�ΪD��

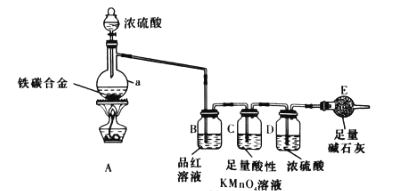
����Ŀ�����Ǵ������������������������ͭ��������֮һ��SnCl4������ýȾ��������������ͼ��ʾװ�ÿ����Ʊ�SnCl4(���ּг�װ�ü���ȥ)��
�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | -33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
(1)���б���ʳ��ˮ������Ϊ___________����װ���������ܵĽ�ˮ��Ϊ________(����a������b��)��
(2)��װ�÷�����Ӧ�����ӷ���ʽΪ________________��
(3)��װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽��װ���ڳ�������ɫ�����ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ�
�ٴٽ�����������Ӧ��
��__________________��
(4)��װ�õ�������_______________��
(5)ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��п����ڼ���Ƿ����SnCl2����__________(����)��
A.FeCl3��Һ(���м���KSCN��Һ) B.��ˮ C.AgNO3��Һ
����Ŀ��A��B��C��D��E��F��G��H��������Ԫ�طֲ���������ͬ�Ķ����ڣ����������ڱ���λ�����£�
A | ||||||
B | C[ | D | ||||
E | F | G | H | |||
��ش��������⣺
��1��д��EԪ�������ڱ��е�λ�� ��
��2��B��D���γ���ԭ�ӷ���X��X�ĵ���ʽΪ ��
��3��E��F��H���γɵļ����ӵİ뾶�ɴ�С��˳��Ϊ �������ӷ��ű�ʾ����
��4��G�������������B�ĵ����ڸ������ܷ����û���Ӧ���仯ѧ��Ӧ����ʽΪ��
��
��5����A��C��D����Ԫ����ɵ�ǿ�����Z����ˮʱ�ܴٽ�ˮ�ĵ��룬���Z��ˮ��ҺpH��7����ԭ���� �������ӷ���ʽ����ʾ����
��6����Y��B��D��E��ɡ���������YΪ����ʹ��ɵ�ȼ�ϵ����ͼ��ʾ��
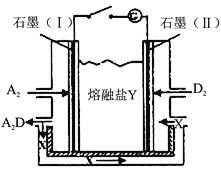
д��ʯī��I���缫�Ϸ����ĵ缫��Ӧʽ ��
��7����������Y��ȼ�ϵ������Դ����ʯīΪ�缫�����һ��Ũ�ȵ�CuSO4��Һ����ɫ��������һ��ʱ�䡣�Ͽ���·������Һ�м���0.1 mol Cu��OH��2����Һ�ָ������֮ǰ�������Ũ�ȣ����������ת�Ƶ��ӵ����ʵ���Ϊ ��