��Ŀ����
8��Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����ij��ѧ�о�С���ͬѧ�ֱ��������ͼ1�ס�����ʾ��ʵ�飮��ش�������⣺��1�����Է�������ͼ��ͨ���۲�������ݵĿ��������ԱȽϵó����ۣ���ͬѧ�����FeCl3��ΪFe2��SO4��3 ��Ϊ�����������������������Ӳ�ͬ��ʵ��ĸ���
��2��������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԣ�ͼ������A������Ϊ��Һ©��������װ�������Եķ����ǹرշ�Һ©���Ļ�������ע����������������һ�Σ���һ������Ƿ�ص�ԭλ��ʵ������Ҫ�����������Dz���40mL���������ʱ�䣮
��3������0.10mol MnO2��ĩ��50mL H2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ��
��Ӧ�ų�$\frac{3}{4}$��������ʱ��ԼΪ2.5 min��
��4������H2O2�ij�ʼ���ʵ���Ũ��0.11 mol•L-1mol/L�����뱣����λ��Ч���֣�

���� ��1�����Է������Ը��ݲ������ݵ������������жϷ�Ӧ�Ŀ�������̽��Ӱ�췴Ӧ���ʵĿ���������ʱͨ����ȡ���Ʊ�����������ʵ�飬ѡ����ʵ����ʣ�
��2������AΪ��Һ©��������װ�������Կ������ȷ���������������Ӧ�Ŀ��������ռ�һ����������壬ʱ�����Ӧ�죻
��3����ͼ2��֪������40mL���壬��ʱΪ2.5min��
��4����ͼ2��֪�����������ڱ�״���µ����Ϊ60mL�����ʵ���Ϊ$\frac{0.06L}{22.4L/mol}$=0.0027mol���پݻ�ѧ����ʽ���������������ʵ�����
��� �⣺��1�����ڶ��Է������Ը��ݲ������ݵ������������жϷ�Ӧ�Ŀ�����Ϊ�Ƚ�Fe3+��Cu2+��H2O2�ֽ�Ĵ�Ч����Ҫ��������������ͬ����FeCl3��Һ�л��������ӣ�CuSO4�к���ͭ���ӣ���������ӿ���Ҳ��Ӱ�췴Ӧ���ʣ��������ţ����Կɽ�FeCl3��ΪFe2��SO4��3��
�ʴ�Ϊ���������ݵĿ��������������Ӳ�ͬ��ʵ��ĸ��ţ�
��2������AΪ��Һ©��������װ�������Եķ����ǣ��رշ�Һ©���Ļ�����������ס��ƿ��ע�����л��������ƶ����ſ��֣������ָ���ԭ��λ�ã�˵�������Ժã�������������Ӧ�Ŀ������Բⶨ�ռ�40mL�����������ʱ�䣬ʱ�����Ӧ�죬
�ʴ�Ϊ����Һ©�����رշ�Һ©���Ļ�������ע����������������һ�Σ���һ������Ƿ�ص�ԭλ���ռ�40mL���������ʱ�䣻
��3����ͼ2��֪������40mL���壬��ʱΪ2.5min���ʴ�Ϊ��2.5��
��4����ͼ��֪�����������ڱ�״���µ����Ϊ60mL�����ʵ���Ϊ$\frac{0.06L}{22.4L/mol}$=0.0027mol���������������ʵ���Ϊ2��0.0027mol=0.0054mol��c��H2O2��=$\frac{0.0054mol}{0.05L}$=0.11mol/L��
�ʴ�Ϊ��0.11mol/L��
���� ����Ӷ��ԺͶ����ĽǶȿ�������Ի�ѧ��Ӧ���ʵ�Ӱ���Լ���������ֽⷴӦ����ʽ�ͻ�ѧ��Ӧ���ʵļ��㣬��Ŀ�Ѷ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�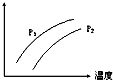 ��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������
��ͼ��ʾ�¶ȡ�ѹǿ�Դ�ƽ��Ŀ��淴Ӧ��2L��g��?2M��g��+N��g����H��0��Ӱ�죨P1��P2��ͼ��y���ʾ�������ǣ�������| A�� | �������L�İٷֺ��� | B�� | ���������ܶ� | ||
| C�� | L��ת���� | D�� | ��������ƽ�������� |
| A�� | ��Һʱ����Һ©���²�Һ����¿ڷų����ϲ�Һ����¿ڷų� | |
| B�� | ʵ������ȡ����ˮ��װ���У��¶ȼ�ˮ����Ӧ��������ƿ��֧�ܿ���ͬһˮƽ�� | |
| C�� | ����ϴ�ķ�����ɳ���Խ� | |
| D�� | ����������ʹNaCl����Һ������ʱ��Ӧ���ȱ߽���ֱ����Һ���� |
| ѡ�� | ��ѧ��Ӧ�����ӷ���ʽ | ���� |
| A | NaClO��Һ��ͨ��������SO2�� ClO-+H2O+SO2=Cl-+SO42-+2H+ | �����Խ����в���������H+ |
| B | �����Ը��������Һ�ζ���� 2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O | ��ȷ |
| C | NH4Al��SO4��2��Һ�е�������NaOH��Һ NH4++OH-=NH3•H2O | ����OH-���Ⱥ�Al3+��Ӧ����Al��OH��3���� |
| D | �ö��Ե缫���CuCl2��Һ 2Cu2++2H2O$\frac{\underline{\;���\;}}{\;}$2Cu+O2��+4H+ | ��ȷ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
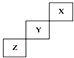
| A�� | X���ʵ��۵��Z�ĵ� | |
| B�� | X��Y��Z����Ԫ���У�X�ķǽ�������ǿ | |
| C�� | Y�⻯����ȶ��Ա�Z���⻯���� | |
| D�� | Y����������ϼ�Ϊ+7 |
| A�� | �ձ� | B�� | ©�� | C�� | ������ | D�� | �ƾ��� |
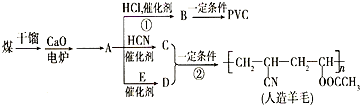 ������������ʯ�ͼ۸����ǣ���úΪԭ���Ʊ�һЩ������Ʒ��ǰ���ֱ����ã���ͼ����úΪԭ��������PVC����������ë�ĺϳ�·�ߣ�
������������ʯ�ͼ۸����ǣ���úΪԭ���Ʊ�һЩ������Ʒ��ǰ���ֱ����ã���ͼ����úΪԭ��������PVC����������ë�ĺϳ�·�ߣ�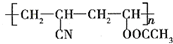 ��
��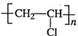 ��
��