��Ŀ����
8����ѧ--ѡ��3���ʽṹ������ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ����Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������η��ӣ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�
��ش��������⣺
��1��������Ԫ���е縺������Ԫ�أ����̬ԭ�ӵļ۵����Ų�ͼΪ
 ��
����2��C�������������Ԫ�طֱ���A�γɵĻ�����е��ɸߵ��͵�˳����HF��HI��HBr��HCl���ѧʽ����������˵ݱ���ɵ�ԭ����HF����֮���γ������ʹ��е�ϸߣ�HCl��HBr��HI�����Ӽ��Է��Ӽ���������ϣ���Է�������Խ���»���Խ�е�Խ�ߣ�
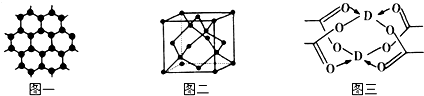
��3��BԪ�ؿ��γɶ��ֵ��ʣ�һ�־���ṹ��ͼһ��ʾ����ԭ�ӵ��ӻ�����Ϊsp2����һ�ֵľ�����ͼ����ʾ���þ����Ŀռ�������Ϊ34%��������λ��Ч���֣�����$\sqrt{3}$=1.732��
��4��DԪ���γɵĵ��ʣ��侧��Ķѻ�ģ��Ϊ�����������ܶѻ���D�Ĵ����ξ���ֲ��ṹ��ͼ�����þ����к��еĻ�ѧ���Ǣ٢ڢۣ���ѡ����ţ���
�ټ��Լ� �ڷǼ��Լ� ����λ�� �ܽ�����
��5����D����������Һ�еμӹ�����ˮ���۲쵽�������������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ����д���������̵����ӷ���ʽCu2++2NH3•H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3•H2O�T[Cu��NH3��4]2++2OH-+4H2O��
���� ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ������AΪ��Ԫ�أ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����������6�����ӣ���BΪ̼Ԫ�أ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����DΪͭԪ�أ����ԭ��������֪��Cֻ�ܴ��ڵ������ڣ�B��C���γ����������ͷ��ӣ���CΪ��Ԫ�أ�
��1������Ԫ���е縺��������Cl�����̬ԭ�ӵļ۵����Ų�Ϊ3s23p5��
��2��HF���Ӽ����������е���ߣ�����±����������Է���������������е����ߣ�
��3��ͼһΪƽ��ṹ�������״�ṹ��̼̼������Ϊ120�㣬ÿ��̼ԭ�Ӷ������3��̼ԭ�ӣ�̼ԭ�Ӳ�ȡsp2�ӻ���
���ݾ�̯�����㾧����Cԭ����Ŀ����̼ԭ��ֱ��Ϊa�����㾧����Cԭ���������̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ�����̼ԭ�ӵ��������Ϊ$\frac{a}{3}$������������ĸ�Ϊ��a+$\frac{a}{3}$����������������ⳤΪx����б��ĸ�Ϊ$\frac{\sqrt{3}}{2}$x���������ĵ��ߵľ���Ϊ$\frac{\sqrt{3}}{2}$x��$\frac{1}{3}$���ٸ��ݹ��ɶ�������x��a�Ĺ�ϵ�������ⳤ=2x��$\frac{\sqrt{2}}{2}$=$\sqrt{2}$x���ټ��㾧������������ռ�������=$\frac{ԭ�������}{�������}$��100%��
��4������CuΪ�����������ܶѻ������ͼ������ͭ����ľֲ��ṹ��ȷ���侧���к��м��Լ����Ǽ��Լ�����λ����
��5������ͭ��Һ�м��백ˮ�����������ͭ��ɫ�����������μӰ�ˮ�������ܽ⣬�õ��İ���ͭ�����ӣ���ҺΪ����ɫ������Һ��
��� �⣺ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ������AΪ��Ԫ�أ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����������6�����ӣ���BΪ̼Ԫ�أ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����DΪͭԪ�أ����ԭ��������֪��Cֻ�ܴ��ڵ������ڣ�B��C���γ����������ͷ��ӣ���CΪ��Ԫ�أ�
��1������Ԫ���е縺��������Cl�����̬ԭ�ӵļ۵����Ų�Ϊ3s23p5�����̬ԭ�ӵļ۵����Ų�ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��2��HF����֮���γ������ʹ���۷е�ϸߣ�HI��HBr��HCl����֮��ֻ�з��»�������Է�������Խ���»���Խ�е�Խ�ߣ����е��ɸߵ��͵�˳����HF��HI��HBr��HCl��
�ʴ�Ϊ��HF��HI��HBr��HCl��HF����֮���γ������ʹ���۷е�ϸߣ�HI��HBr��HCl����֮��ֻ�з��»�������Է�������Խ���»���Խ�е�Խ�ߣ�
��3��ͼһΪƽ��ṹ�������״�ṹ��̼̼������Ϊ120�㣬ÿ��̼ԭ�Ӷ������3��̼ԭ�ӣ�̼ԭ�Ӳ�ȡsp2�ӻ���
һ�������к�̼ԭ����Ϊ8��$\frac{1}{8}$+6��$\frac{1}{2}$+4=8����̼ԭ��ֱ��Ϊa��������Cԭ�������=8��$\frac{4}{3}$�У�$\frac{a}{2}$��3��̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ�����̼ԭ�ӵ��������Ϊ$\frac{a}{3}$������������ĸ�Ϊ��a+$\frac{a}{3}$��=$\frac{4a}{3}$��������������ⳤΪx����б��ĸ�Ϊ$\frac{\sqrt{3}}{2}$x���������ĵ��ߵľ���Ϊ$\frac{\sqrt{3}}{2}$x��$\frac{1}{3}$���ٸ��ݹ��ɶ�������$\frac{4a}{3}$��2+��$\frac{\sqrt{3}}{2}$x��$\frac{1}{3}$��2=��$\frac{\sqrt{3}}{2}$x��2��������x=$\frac{2\sqrt{6}}{3}$a���ʾ����ⳤ=$\frac{2\sqrt{6}}{3}$a��$\sqrt{2}$=$\frac{4\sqrt{3}}{3}$a�������Ϊ��$\frac{4\sqrt{3}}{3}$��3�������ռ�������={[8��$\frac{4}{3}$�У�$\frac{a}{2}$��3]�£�$\frac{4\sqrt{3}}{3}$��3}��100%��34%��
�ʴ�Ϊ��sp2��34%��
��4������CuΪ�����������ܶѻ������ͼ������ͭ����ľֲ��ṹ��ȷ���侧���к��м��Լ����Ǽ��Լ�����λ����
�ʴ�Ϊ�������������ܶѻ����٢ڢۣ�
��5������ͭ��Һ�м��백ˮ�������ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ���йط�Ӧ�����ӷ���ʽΪCu2++2NH3•H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3•H2O�T[Cu��NH3��4]2++2OH-+4H2O���ʴ�Ϊ�������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ��Cu2++2NH3•H2O�TCu��OH��2��+2NH4+��Cu��OH��2+4NH3•H2O�T[Cu��NH3��4]2++2OH-+4H2O��
���� �����Ƕ����ʽṹ�Ŀ��飬��Ŀ�ۺ��Խϴ��漰Ԫ���ƶϡ���������Ų�������ṹ�뻯ѧ�����ӻ������������������ȣ��Ƕ�ѧ���ۺ������Ŀ��飬��3���м���Ϊ�״��㡢�Ѷȣ���Ҫѧ���߱�һ���Ŀռ���������ѧ�����������Ѷ��еȣ�

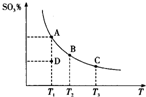 ��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��������Ĵ�����ԭ��Ϊ2SO2��g��+O2��g��?2SO3��g������Ӧ�����ϵ��ƽ��״̬ʱSO3�İٷֺ������¶ȵĹ�ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��D��ʱ����Ӧ������� | |
| B�� | ��Ӧ2SO2��g��+O2��g��?2SO3��g���ġ�H��0 | |
| C�� | ��B��C���ƽ�ⳣ���ֱ�ΪKB��KC����KB��KC | |
| D�� | ���º�ѹ����ƽ����ϵ��ͨ�뺤����ƽ�������ƶ� |
| A�� | ��С�մ���Һ�μ��������HCO3-+H+�T+H2O+CO2�� | |
| B�� | ��Ư��Һ���չ���SO2��SO2+H2O+ClO-=2H++SO42-+Cl- | |
| C�� | ��������ϩ����ͨ������KMnO4��Һ��CH2=CH2+4H++MnO4-�TC2O42-+Mn2++4H2O | |
| D�� | ��ͭ˿����ϡ�����У�3Cu+8H++2NO3-�T3Cu2++2NO��+4H2O |
| A�� | �ۢݢ� | B�� | �ܢݢ� | C�� | �ܢݢ� | D�� | �ۢޢ� |
| A�� | ��A��ʾ�ķ�Ӧ������0.4mol•��L•min��-1 | |
| B�� | ��2minĩ�ķ�Ӧ���ʣ���B��ʾ��0.3mol•��L•min��-1 | |
| C�� | �ֱ���B��C��D��ʾ��Ӧ�����ʣ������3��2��1 | |
| D�� | ����2min����B��C��ʾ�ķ�Ӧ���ʵ�ֵ������С�� |
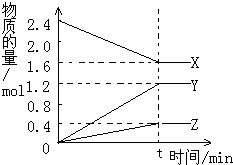 ��2L������3�����ʼ���з�Ӧ��X��Y��Z�����ʵ�����ʱ��ı仯������ͼ����Ӧ��tʱ����ƽ�⣬��ͼ��ʾ��
��2L������3�����ʼ���з�Ӧ��X��Y��Z�����ʵ�����ʱ��ı仯������ͼ����Ӧ��tʱ����ƽ�⣬��ͼ��ʾ��