��Ŀ����
A��B��C��D��E�����������ɳ���Ԫ����ɣ�A������Ԫ����ɣ���A������Ԫ�ص�������Ϊ7��8��A��B�ڸ��������·�Ӧ����C��D��CΪ��ɫ���壬�ڱ�״���µ��ܶ�ԼΪ2.86g?L��1����AΪԭ������E��

��д��A�Ļ�ѧʽ��
��A��B�ķ�Ӧ�У���ԭ���������������ʵ���֮��Ϊ ��
��ij����������ͼ��ʾװ�����õ绯ѧԭ������E����N�缫���� ����д��M�缫�ĵ缫��Ӧʽ�� ��
�ȵ�ͼ�е�C��O2������̿����ʱ��Ҳ�ܷ�����Ӧ����F��Ϊ�о��˷�Ӧ����C��O2�������Ϊ2L�ĺ����ܱ������У���500��ʱ������Ӧ����Ӧ�����е������仯����ͼ��ʾ����Ӧ�����ʵ�����ʱ��ı仯���±���ʾ��

�ٸ÷�Ӧ�Ħ�H 0��Ӧ����ʼ��2min�ڵ�ƽ����Ӧ����v(C)�� ��
500��ʱ���˷�Ӧ��ѧƽ�ⳣ��K��ֵΪ ��
�ڵ�6minʱ�����������ٳ���0.002molO2��ͬʱ����Ӧ��ϵ���¶ȸı�ΪT����Ӧ��10minʱ���´ﵽƽ�⣬��ʱ���c(C)��0.006mol?L��1�����¶�T �������������500�档

��д��A�Ļ�ѧʽ��
��A��B�ķ�Ӧ�У���ԭ���������������ʵ���֮��Ϊ ��
��ij����������ͼ��ʾװ�����õ绯ѧԭ������E����N�缫���� ����д��M�缫�ĵ缫��Ӧʽ�� ��
�ȵ�ͼ�е�C��O2������̿����ʱ��Ҳ�ܷ�����Ӧ����F��Ϊ�о��˷�Ӧ����C��O2�������Ϊ2L�ĺ����ܱ������У���500��ʱ������Ӧ����Ӧ�����е������仯����ͼ��ʾ����Ӧ�����ʵ�����ʱ��ı仯���±���ʾ��

�ٸ÷�Ӧ�Ħ�H 0��Ӧ����ʼ��2min�ڵ�ƽ����Ӧ����v(C)�� ��
500��ʱ���˷�Ӧ��ѧƽ�ⳣ��K��ֵΪ ��
�ڵ�6minʱ�����������ٳ���0.002molO2��ͬʱ����Ӧ��ϵ���¶ȸı�ΪT����Ӧ��10minʱ���´ﵽƽ�⣬��ʱ���c(C)��0.006mol?L��1�����¶�T �������������500�档
�� FeS2 �� 11��4�� ��������SO2��2e����2H2O��SO42����4H����
�Ȣ٣��� 0.018mol��L��1��min��1�� 8000 �ڣ�
�Ȣ٣��� 0.018mol��L��1��min��1�� 8000 �ڣ�
��1���ڱ�״���µ��ܶ�ԼΪ2.86g?L��1����C����Է���������2.86��22.4��64������C��SO2�����Ը���A����ɿ�֪��A��FeS2��
��2��FeS2�ڷ�Ӧ��ʧȥ3-2����4��1����2��11�����ӣ�������ֻ�ܵõ�4�����ӣ����Ը��ݵ��ӵ�ʧ�غ��֪����ԭ���������������ʵ���֮��Ϊ11��4��
��3��ԭ����и���ʧȥ���ӣ������õ����ӣ�N��ͨ������������N��������SO2��M��ͨ�룬�缫��ӦʽΪSO2��2e����2H2O��SO42����4H����
��4���ٸ���ͼ���֪����Ӧ�����������������������������Ƿ��ȷ�Ӧ����HС��0��2min��SO2�ı仯����0.108mol��0.036mol��0.072mol�������䷴Ӧ������0.072mol��2L��2min��0.018mol��L��1��min��1��ƽ��ʱSO2��������Ũ�ȷֱ���0.006mol/L��0.008mol/L������SO3��0.054mol/L��0.006mol/L��0.048mol/L������ƽ�ⳣ��Ϊ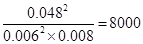
����������Ũ��ƽ��������Ӧ�����ƶ���������ƽ���SO2Ũ�Ȳ��䣬˵���ı��¶�Ӧ���������¶ȣ�ƽ�����淴Ӧ�����ƶ���
��2��FeS2�ڷ�Ӧ��ʧȥ3-2����4��1����2��11�����ӣ�������ֻ�ܵõ�4�����ӣ����Ը��ݵ��ӵ�ʧ�غ��֪����ԭ���������������ʵ���֮��Ϊ11��4��
��3��ԭ����и���ʧȥ���ӣ������õ����ӣ�N��ͨ������������N��������SO2��M��ͨ�룬�缫��ӦʽΪSO2��2e����2H2O��SO42����4H����
��4���ٸ���ͼ���֪����Ӧ�����������������������������Ƿ��ȷ�Ӧ����HС��0��2min��SO2�ı仯����0.108mol��0.036mol��0.072mol�������䷴Ӧ������0.072mol��2L��2min��0.018mol��L��1��min��1��ƽ��ʱSO2��������Ũ�ȷֱ���0.006mol/L��0.008mol/L������SO3��0.054mol/L��0.006mol/L��0.048mol/L������ƽ�ⳣ��Ϊ
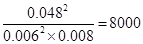
����������Ũ��ƽ��������Ӧ�����ƶ���������ƽ���SO2Ũ�Ȳ��䣬˵���ı��¶�Ӧ���������¶ȣ�ƽ�����淴Ӧ�����ƶ���

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ




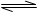 2NH3��g���ġ�H= ��
2NH3��g���ġ�H= ��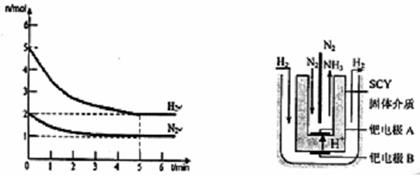
 H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���
H2(g) + CO2(g)��ƽ�ⳣ�����¶ȵı仯���±���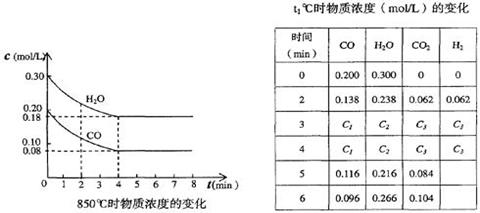
 1/2N2O4(g) ��H = ��26.35 kJ��mol��1
1/2N2O4(g) ��H = ��26.35 kJ��mol��1 CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1��
CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1�� CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1





 ��
�� 2CA3(g)������Ӧ�ﵽƽ��ʱ���ϸı�����(���ı�A2��C2��CA3����)����ͼ
2CA3(g)������Ӧ�ﵽƽ��ʱ���ϸı�����(���ı�A2��C2��CA3����)����ͼ