Ő‚ńŅńŕ»›
°ĺŐ‚ńŅ°ŅA°ĘB°ĘC°ĘD°ĘE°ĘFő™«įňń÷‹∆ŕ‘≠◊”–Ú ż“ņīő‘ŲīůĶńŃý÷÷‘™ňō£¨A°ĘC°ĘD‘≠◊”ĺý”–ŃĹłŲőī≥…∂‘ĶÁ◊”£¨A°ĘB°ĘCÕ¨÷‹∆ŕ£¨A”ŽD°ĘB”ŽF∑÷ĪūÕ¨÷ų◊Ś£¨E «…ķĽÓ÷–”√ŃŅ◊ÓīůĶńĹū Ű°£«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©EĪ»ĹŌő»∂®ĶńņŽ◊”ļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺ_____________________________£ģ
£®2£©A°ĘB°ĘCĶńĶŕ“ĽĶÁņŽń‹”…–°ĶĹīůĶńň≥–Úő™_________________![]() ”√‘™ňō∑ŻļŇĪŪ ĺ
”√‘™ňō∑ŻļŇĪŪ ĺ![]() °ĘB°ĘCĶńľÚĶ•«‚ĽĮőÔ÷–◊Ó“◊∆ŻĽĮĶńőÔ÷ ĶńĽĮ—ß Ĺ___________£ģ
°ĘB°ĘCĶńľÚĶ•«‚ĽĮőÔ÷–◊Ó“◊∆ŻĽĮĶńőÔ÷ ĶńĽĮ—ß Ĺ___________£ģ
£®3£©C”ŽD–ő≥…ĶńőÔ÷ ĶńĺßŐŚņŗ–Õ «____________£¨IT≤ķ“Ķ÷–ł√ĺßŐŚ”√”ŕ…ķ≤ķ____________£ģ
£®4£©”…A°ĘB°ĘC»ż÷÷‘™ňō÷–Ķń“Ľ÷÷ĽÚŃĹ÷÷‘™ňō–ő≥…Ķń∑÷◊”÷–£¨”–ĶńĽ•ő™Ķ»ĶÁ◊”ŐŚ£¨–ī≥Ų∆š÷–“Ľ◊ťĶ»ĶÁ◊”ŐŚĶńĽĮ—ß Ĺ£ļ______![]() ≤Ę–ī≥Ų∂‘”¶ĶńĹŠĻĻ Ĺ_______________£ģ
≤Ę–ī≥Ų∂‘”¶ĶńĹŠĻĻ Ĺ_______________£ģ
£®5£©BĶńĶ•÷ ĺßįŻ”Ž![]() ĶńŌŗň∆£¨‘Ú“ĽłŲĺßįŻ÷–ļ¨BĶń‘≠◊”łŲ żő™____
ĶńŌŗň∆£¨‘Ú“ĽłŲĺßįŻ÷–ļ¨BĶń‘≠◊”łŲ żő™____![]() ”Ž«‚–ő≥…Ķń∑÷◊”Ņ’ľšĻĻ–Õ «_______£ģ
”Ž«‚–ő≥…Ķń∑÷◊”Ņ’ľšĻĻ–Õ «_______£ģ
£®6£©°įŇÝň™°Ī «“Ľ÷÷ļ¨C£¨FĶńĽĮļŌőÔ£¨∆š∑÷◊”ĹŠĻĻ»ÁÕľ1ňý ĺ£¨ł√ĽĮļŌőÔĶń∑÷◊” Ĺő™F4C6£¨F‘≠◊”≤…»°______‘”ĽĮ£ĽC£¨D£¨E◊ť≥…ĶńĽĮļŌőÔĶńĺßįŻ»ÁÕľ2£¨∆šĺßįŻ≤ő żő™a pm£¨‘Ú∆š√‹∂»ő™___________________g/cm3£®Ń–≥Ų Ĺ◊”ľīŅ…£¨įĘ∑ŁŔ§Ķ¬¬ř≥£ żő™NAmol-1£©°£

°ĺīūįł°Ņ ![]() ĽÚ
ĽÚ![]()
![]()
![]() ‘≠◊”ĺßŐŚ Ļ‚ĶľŌňő¨
‘≠◊”ĺßŐŚ Ļ‚ĶľŌňő¨ ![]() ļÕCO
ļÕCO ![]() ļÕ
ļÕ![]() 8 »żĹ«◊∂–ő sp3
8 »żĹ«◊∂–ő sp3 ![]()
°ĺĹ‚őŲ°ŅA°ĘB°ĘC°ĘD°ĘE°ĘFő™«įňń÷‹∆ŕ‘≠◊”–Ú ż“ņīő‘ŲīůĶńŃý÷÷‘™ňō£¨A°ĘC°ĘD‘≠◊”ĺý”–ŃĹłŲőī≥…∂‘ĶÁ◊”£¨‘ÚA «÷ ◊” żő™6ĶńC£¨C «÷ ◊” żő™8ĶńO£¨Dő™÷ ◊” żő™14ĶńSi£Ľ∂ÝA°ĘB°ĘCÕ¨÷‹∆ŕ£¨‘ÚB «N£ĽA”ŽD°ĘB”ŽF∑÷ĪūÕ¨÷ų◊Ś£¨‘ÚF «As£ĽE «…ķĽÓ÷–”√ŃŅ◊ÓīůĶńĹū Ű£¨‘ÚEő™Fe°£◊Ř…Ōňý Ų£¨Aő™C£¨Bő™N£¨Cő™O£¨Dő™Si£¨Eő™Fe£¨Fő™As°£
£®1£©FeĪ»ĹŌő»∂®ĶńņŽ◊” «Fe3+,∆šļňÕ‚ĶÁ◊”ŇŇ≤ľ Ĺő™![]() ĽÚ
ĽÚ![]() °£
°£
£®2£©Õ¨÷‹∆ŕ‘™ňōňś‘≠◊”–Ú żĶń‘Ųīů£¨Ķŕ“ĽĶÁņŽń‹≥ ‘Ųīů«ų ∆£¨ĶęNĶń2pń‹ľ∂»›ń…3łŲĶÁ◊”£¨ő™įŽ≥š¬ķ◊īŐ¨£¨ń‹ŃŅĹŌĶÕ£¨∆šĶŕ“ĽĶÁņŽń‹łŖ”ŕO£¨Ļ Ķŕ“ĽĶÁņŽń‹”…–°ĶĹīůĶńň≥–Úő™£ļ![]() £Ľ
£Ľ![]() °ĘB°ĘCĶńľÚĶ•«‚ĽĮőÔ÷–◊Ó“◊∆ŻĽĮĶńőÔ÷ ĺÕ «∑–Ķ„◊ÓĶÕĶń£¨∆š÷–H2OļÕNH3÷–ĺýīś‘ŕ«‚ľŁ£¨∑–Ķ„ĹŌłŖ£¨ňý“‘◊Ó“◊∆ÝĽĮĶńőÔ÷ ő™CH4°£
°ĘB°ĘCĶńľÚĶ•«‚ĽĮőÔ÷–◊Ó“◊∆ŻĽĮĶńőÔ÷ ĺÕ «∑–Ķ„◊ÓĶÕĶń£¨∆š÷–H2OļÕNH3÷–ĺýīś‘ŕ«‚ľŁ£¨∑–Ķ„ĹŌłŖ£¨ňý“‘◊Ó“◊∆ÝĽĮĶńőÔ÷ ő™CH4°£
£®3£©C”ŽD–ő≥…ĶńőÔ÷ «∂Ģ—űĽĮĻŤ£¨ Ű”ŕ‘≠◊”ĺßŐŚ£¨IT≤ķ“Ķ÷–ł√ĺßŐŚ”√”ŕ…ķ≤ķĻ‚ĶľŌňő¨°£
£®4£©”…C°ĘN°ĘO»ż÷÷‘™ňō÷–Ķń“Ľ÷÷ĽÚŃĹ÷÷‘™ňō–ő≥…Ķń∑÷◊”÷–Ľ•ő™Ķ»ĶÁ◊”ŐŚ”–![]() ļÕCO£¨∂‘”¶ĶńĹŠĻĻ Ĺ
ļÕCO£¨∂‘”¶ĶńĹŠĻĻ Ĺ![]() ļÕ
ļÕ![]() °£
°£
£®5£©N2ĶńĺßįŻ”Ž![]() ĶńŌŗň∆£¨ňý“‘ĺßįŻ÷–Ķ™∆Ý∑÷◊”ĶńłŲ żő™£ļ8
ĶńŌŗň∆£¨ňý“‘ĺßįŻ÷–Ķ™∆Ý∑÷◊”ĶńłŲ żő™£ļ8![]() +6
+6![]() =4£¨‘Ú“ĽłŲĺßįŻ÷–ļ¨NĶń‘≠◊”łŲ żő™2
=4£¨‘Ú“ĽłŲĺßįŻ÷–ļ¨NĶń‘≠◊”łŲ żő™2![]() As”Ž«‚–ő≥…Ķń∑÷◊”ő™AsH3,∆šŅ’ľšĻĻ–Õņŗň∆”ŕNH3,ő™»żĹ«◊∂–ő°£
As”Ž«‚–ő≥…Ķń∑÷◊”ő™AsH3,∆šŅ’ľšĻĻ–Õņŗň∆”ŕNH3,ő™»żĹ«◊∂–ő°£
£®6£©—ű‘≠◊”ń‹–ő≥…2łŲĻ≤ľŘľŁ£¨As–ő≥…3łŲĻ≤ľŘľŁ£¨”…ÕľŅ…÷™∑÷◊”÷–O‘≠◊” żő™6£¨As‘≠◊” żő™4£¨ňý“‘∆šĽĮ—ß Ĺő™As4O6£ĽAs◊ÓÕ‚≤„”–5łŲĶÁ◊”£¨–ő≥…3łŲĻ≤ľŘľŁľī![]() ľŁĶÁ◊”∂‘ żő™3£¨ŃŪÕ‚ĽĻ”–1łŲĻ¬ĶÁ◊”∂‘£¨ňý“‘AsĶń‘”ĽĮ∑Ĺ Ĺő™sp3£Ľ‘ŕł√ĺßįŻĹŠĻĻ÷–£¨ļ¨”–O°ĘSi°ĘFe£¨∆š÷–ļ¨Fe‘≠◊” żńŅő™8
ľŁĶÁ◊”∂‘ żő™3£¨ŃŪÕ‚ĽĻ”–1łŲĻ¬ĶÁ◊”∂‘£¨ňý“‘AsĶń‘”ĽĮ∑Ĺ Ĺő™sp3£Ľ‘ŕł√ĺßįŻĹŠĻĻ÷–£¨ļ¨”–O°ĘSi°ĘFe£¨∆š÷–ļ¨Fe‘≠◊” żńŅő™8![]() =2£¨ļ¨Si
=2£¨ļ¨Si![]() +6
+6![]() =4£¨ĽĮ—ß Ĺő™Fe2SiO4£¨∆šń¶∂Ż÷ ŃŅő™204g/mol£¨ĺßįŻĶń√‹∂»ő™
=4£¨ĽĮ—ß Ĺő™Fe2SiO4£¨∆šń¶∂Ż÷ ŃŅő™204g/mol£¨ĺßįŻĶń√‹∂»ő™![]() =
=![]() =
=![]() g
g![]() cm-3,Ļ īūįłő™£ļ
cm-3,Ļ īūįłő™£ļ![]() °£
°£

°ĺŐ‚ńŅ°Ņ‘ŕ25 °ś Ī£¨√‹Ī’»›∆ų÷–X°ĘY°ĘZ»ż÷÷∆ÝŐŚĶń≥ű ľŇ®∂»ļÕ∆Ĺļ‚Ň®∂»»ÁŌ¬ĪŪ£ļ
őÔ÷ | X | Y | Z |
≥ű ľŇ®∂»/mol°§L£≠1 | 0.1 | 0.2 | 0 |
∆Ĺļ‚Ň®∂»/mol°§L£≠1 | 0.05 | 0.05 | 0.1 |
Ō¬Ń–ňĶ∑®’ż»∑Ķń «
A. ∑ī”¶Ņ…ĪŪ ĺő™£ļX(g)£ęY(g) ![]() 2Z(g)
2Z(g)
B. ∑ī”¶īÔĶĹ∆Ĺļ‚ Ī£¨XĶń◊™ĽĮ¬ ő™60%
C. ‘ŕīňŐűľĢŌ¬£¨ł√∑ī”¶Ķń∆Ĺļ‚≥£ żő™K=1600
D. ∆šňŻŐűľĢ≤ĽĪš Ī£¨‘Ųīů—Ļ«Ņ∆Ĺļ‚∑Ę…ķ“∆∂Į£¨‘ŔīÔ–¬∆Ĺļ‚£¨∆Ĺļ‚≥£ ż‘Ųīů
°ĺŐ‚ńŅ°Ņ≥£ő¬Ō¬£¨ń≥Õ¨—ßĹę—őňŠ”ŽįĪňģĶ»ŐŚĽżĽžļŌ£¨ŃĹ÷÷»‹“ļĶńŇ®∂»ļÕĽžļŌļůňýĶ√»‹“ļĶńpH»ÁŌ¬ĪŪ£ļ
Ķ—ťĪŗļŇ | įĪňģŇ®∂»/mol°§L-1 | —őňŠŇ®∂»/mol°§L-1 | ĽžļŌ»‹“ļpH |
ĘŔ | 0.1 | 0.1 | pH£Ĺ5 |
Ęŕ | c | 0.2 | pH£Ĺ7 |
ĘŘ | 0.2 | 0.1 | pH£ĺ7 |
«ŽĽōīūŌ¬Ń–ő Ő‚£ļ
£®1£©ĘŔ÷–ňýĶ√ĽžļŌ»‹“ļc(OH-)£Ĺ______ mol°§L-1°£
£®2£©Ęŕ÷–£¨c______0.2£®ŐÓ°į>°Ī°į<°ĪĽÚ°į£Ĺ°Ī£©°£
£®3£©ĘŘ÷–ňýĶ√ĽžļŌ»‹“ļ£¨łųņŽ◊”Ň®∂»”…īůĶĹ–°Ķńň≥–Ú «_______°£
£®4£©ĘŔ°ĘĘŘňý”√įĪňģ÷–Ķń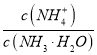 £ļĘŔ_______ĘŘ£®ŐÓ°į>°Ī°į<°ĪĽÚ°į£Ĺ°Ī£©°£
£ļĘŔ_______ĘŘ£®ŐÓ°į>°Ī°į<°ĪĽÚ°į£Ĺ°Ī£©°£