题目内容
【题目】现欲配制500 mL 0.040 mol·L-1的K2Cr2O7溶液。
(1)所需的仪器有:托盘天平、药匙、烧杯、________、________、________。(在横线上填写所缺仪器的名称)
(2)在溶液的配制过程中,有以下基本实验步骤,正确的操作顺序是(填写操作步骤的代号,每个操作步骤只用一次)________。
①颠倒摇匀 ②定容 ③洗涤 ④溶解 ⑤转移 ⑥称量
(3)用托盘天平称取K2Cr2O7固体的质量为________ g。
(4)下列操作使最后实验结果偏小的是________(填序号)。
A.加水定容时俯视刻度线 B.转移前,容量瓶中含有少量蒸馏水未干燥
C.未洗涤烧杯内壁和玻璃棒 D.摇匀后发现凹液面低于刻度线又加水补上
(5)定容时,如果不小心加水超过了刻度线,则处理的方法是________ 。
【答案】玻璃棒 500 mL容量瓶 胶头滴管 ⑥④⑤③②① 5.9 C D 重新配制
【解析】
(1)根据配制的操作步骤来分析需要的仪器;
(2)配制步骤是计算、称量、溶解、转移、洗涤、定容、摇匀、装瓶;
(3)根据n = cV、m = nM计算出500mL 0.040 mol·L-1的K2Cr2O7溶液中含有溶质K2Cr2O7的质量;
(4)根据实验操作对c = ![]() 的影响进行误差分析;
的影响进行误差分析;
(5)配制过程中的操作失误,能补救就补救,不能补救就需重新配制;
(1)操作步骤有计算、称量、溶解、转移、洗涤、定容、摇匀等操作,一般用托盘天平称量,用药匙取用药品,在烧杯中溶解(可用量筒量取水加入烧杯),并用玻璃棒搅拌,加速溶解。冷却后转移到500mL容量瓶中,并用玻璃棒引流,洗涤烧杯、玻璃棒23次,并将洗涤液移入容量瓶中,加水至液面距离刻度线12cm时,改用胶头滴管滴加,最后定容颠倒摇匀。所以所需仪器除托盘天平、药匙、烧杯以外,还缺玻璃棒、500mL容量瓶和胶头滴管,
故答案为:玻璃棒、500mL容量瓶和胶头滴管;
(2)当计算完所需固体的质量以后,配制一定浓度的溶液基本操作步骤为称量、溶解、转移、洗涤、定容、摇匀等过程,所以正确的顺序为:⑥④⑤③②①,
故答案为:⑥④⑤③②①;
(3)溶液中溶质K2Cr2O7的物质的量为:n = cV = 0.040 mol·L-1![]() 0.5 L = 0.020 mol,又K2Cr2O7的摩尔质量为:M = (39
0.5 L = 0.020 mol,又K2Cr2O7的摩尔质量为:M = (39![]() 2+52
2+52![]() 2+16
2+16![]() 7 )g/mol= 294 g/mol,再根据m = nM = 0. 020 mol
7 )g/mol= 294 g/mol,再根据m = nM = 0. 020 mol![]() 294 g/mol = 5.88 g,实际操作时托盘天平只能精确到小数点后一位,因此按四舍五入原则用托盘天平称量的质量为5.9 g,
294 g/mol = 5.88 g,实际操作时托盘天平只能精确到小数点后一位,因此按四舍五入原则用托盘天平称量的质量为5.9 g,
故答案为:5.9 ;
(4)A.定容时俯视刻度线,导致配制的溶液体积偏小,则配制的溶液浓度偏高,不符合题意,故A项错误;
B.转移前,容量瓶中含有少量蒸馏水未干燥,对测试结果无影响,因为转移以后也要加水至刻度线1-2cm再定容,只要定容操作无误,原有的少量蒸馏水对浓度无影响,故B项不符合题意;
C. 未洗涤烧杯内壁和玻璃棒,会使溶质质量减少,溶质物质的量减少,溶液浓度偏低,故C项符合题意;
D. 摇匀后发现凹液面低于刻度线又加水补上,相当于稀释了原溶液,则会使溶液浓度偏低,故D项符合题意,
故答案为:CD;
(5)定容是加水超过了刻度线,是无法补救的,故应重新配制,
故答案为:重新配制;

 金钥匙试卷系列答案
金钥匙试卷系列答案【题目】为了探究氨气及氨水的还原性,某兴趣小组同学设计了以下探究活动。
I.探究氨气的还原性
该兴趣小组同学利用以下装置(夹持,加热仪器略)探究氯气与氨气的反应,其中A、F分别为氯气和氨气的发生装置,B为纯净干燥的氯气与氨气反应的装置。
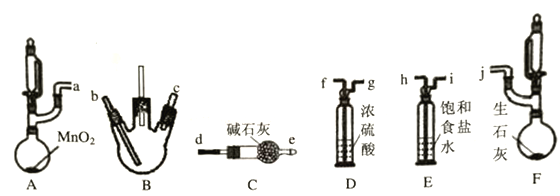
请回答下列问题:
(1)上述装置接口的连接顺序为a接h、i接f、g接___、____接___、____接j,其中装置D的作用是____________。
(2)若氨气足量,装置B中出现的现象为____________。
II.探究氨水的还原性
该兴趣小组同学探究不同条件下高锰酸钾溶液与氨水的反应,实验如下:
实验 | 操作 | 现象 |
① | 取2mL.0.01mol/LKMnO4溶液于试管中,加入新开封1mL浓氨水,加入半滴管蒸馏水,振荡,用橡皮塞塞住。 | 产生棕褐色物质(MnO2),约10min后溶液紫红色变浅 |
② | 取2mL0.01mol/LKMnO4溶液于试管中,加入新开封1mL浓氨水,加入半滴管1:5的硫酸,振荡,用橡皮塞塞住。 | 产生棕褐色物质(MnO2),溶液紫红色立刻变浅,约2min后溶液紫红色完全退去 |
③ | 取2mL0.1mol/LKMnO4溶液于试管中,加入新开封ImL浓氨水,加入半滴管蒸馏水,振荡,用橡皮塞塞住。 | 产生棕褐色物质(MnO2),约10min后溶液紫红色变浅 |
④ | 取2mL0.1mol/LKMnO4溶液于试管中,加入新开封1mL浓氨水,加人半滴管1:5的硫酸,振荡,用橡皮塞塞住。 | 产生棕褐色物质(MnO2),溶液紫红色立刻变浅,约5min后溶液紫红色完全退去 |
(3)实验①中氧化产物为N2,写出该反应的离子方程式:_________。
(4)实验①②说明________________。
(5)实验②比实验④反应速率_____(填“快“或“慢”),原因是_________。
(6)1:5的硫酸溶液(密度为ρ2g·cm-3),可用质量分数为98%的浓硫酸(密度为ρ1g·cm-3)和
蒸馏水按体积比1:5配成,则该1:5的硫酸溶液的物质的量浓度为_____mol/L。(用含ρ1、ρ2的式子表示)
(7)由实验I、II可得出的结论是____________________。