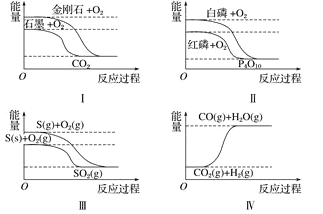
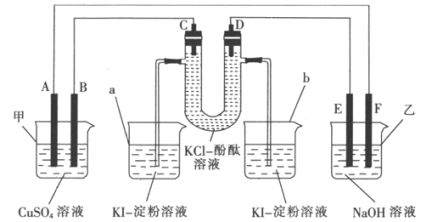
��Ŀ����
����Ŀ��һ�������£��������ʵ�����A��B�������2 L���ܱ������У��������·�Ӧ��3A(g)��B(g) ![]() mC(g)��2D(g)������5 min��Ӧ�ﵽƽ�⡣��ʱ��ã�D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��1��3��C�ķ�Ӧ������0.1 mol��L��1��min��1����
mC(g)��2D(g)������5 min��Ӧ�ﵽƽ�⡣��ʱ��ã�D��Ũ��Ϊ0.5 mol/L��c(A)��c(B)��1��3��C�ķ�Ӧ������0.1 mol��L��1��min��1����
��m��ֵΪ���٣�_____________
��A��5 minĩ��Ũ�ȡ�__________
��B��ƽ��ת���ʡ�___________
�ܸ÷�Ӧ��ƽ�ⳣ����___________
���𰸡�m=2 0.25 mol��L��1 25% ![]()
��������
��D��Ũ��Ϊ0.5mol��L��1����D�ķ�Ӧ����Ϊ![]() =0.1 mol��L��1��min��1�����ݻ�ѧ��Ӧ����֮�ȵ��ڼ�����֮��0.1 mol��L��1��min��1��0.1 mol��L��1��min��1=m:2����m=2��
=0.1 mol��L��1��min��1�����ݻ�ѧ��Ӧ����֮�ȵ��ڼ�����֮��0.1 mol��L��1��min��1��0.1 mol��L��1��min��1=m:2����m=2��
���跴Ӧ��ʼǰ����������A��B���ʵ���Ũ��Ϊa����
3A(g)��B(g) ![]() 2C(g)��2D(g)
2C(g)��2D(g)
��ʼ��Ũ�ȣ��� a a 0 0
���ģ�Ũ�ȣ��� 0.75 0.25 0.5 0.5
ƽ�⣨Ũ�ȣ���(a��0.75) (a��0.25) 0.5 0.5
��Ϊc(A)�Uc(B)=1:3������a-0.75��:��a-0.25��=1:3�����a=1mol/L����A��B���ʵ���Ũ�ȶ�Ϊ1mol/L��
A��5 minĩ��Ũ��Ϊ��a��0.75��mol/L =1mol/L-0.75mol/L=0.25mol/L��
��B��ƽ��ת����Ϊ![]() ��
��
�ܸ÷�Ӧ��ƽ�ⳣ��K=![]() =
=![]() ��
��

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�