��Ŀ����
19����֪ij�л���AΪ����������Է�������Ϊ70��������֮���ת����ϵ����ͼ��ʾ������B��D��E�Ľṹ�о�����2�������Һ˴Ź���������4���壮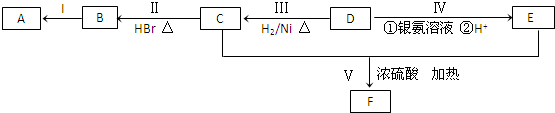
��1��A�ķ���ʽΪC5H10��I�ķ�Ӧ����ΪNaOH���Ҵ���Һ������
��2��д����Ӧ���ТٵĻ�ѧ����ʽ
 ��
����3��д����Ӧ���Ļ�ѧ����ʽ
 ��
����4��E�ж���ͬ���칹�壬д���������������ʵĽṹ��ʽ��
���ܷ���������Ӧ������������Ʒ�Ӧ���������������ܷ�����ȥ��Ӧ��
����������������ˮ�⣬������ˮ�������Է���������ͬ����
 ����
���� ��
�� ��
��
���� �л���AΪ����������ͼ��������Է�������Ϊ70��������Cԭ�������ĿΪ$\frac{70}{12}$=5��10����A�ķ���ʽΪC5H10��D�����������ӳɷ�Ӧ���ܹ���������Һ��Ӧ����D����-CHO��E����-COOH��C����-OH��C��HBr����ȡ����Ӧ����±����B����Bת��ΪA������ȥ��Ӧ����AΪϩ��������B��D��E�Ľṹ�о�����2��-CH3�����ǵĺ˴Ź��������о�����4���壬��DΪ��CH3��2CHCH2CHO��CΪ��CH3��2CHCH2CH2OH��BΪ��CH3��2CHCH2CH2Br��AΪ��CH3��2CHCH=CH2��EΪ��CH3��2CHCH2COOH��C��E����������Ӧ����FΪ��CH3��2CHCH2COOCH2CH2CH��CH3��2���ݴ˽��
��� �⣺��1���л���AΪ����������ͼ��������Է�������Ϊ70��������Cԭ�������ĿΪ$\frac{70}{12}$=5��10����A�ķ���ʽΪC5H10��BΪ��CH3��2CHCH2CH2Br��AΪ��CH3��2CHCH=CH2����Bת��ΪA���ȴ�������ȥ��Ӧ������NaOH���Ҵ���Һ�м��ȣ��ʴ�Ϊ��C5H10��NaOH���Ҵ���Һ�����ȣ�
��2����Ӧ���ТٵĻ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��3����Ӧ���Ļ�ѧ����ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
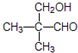
��4��EΪ��CH3��2CHCH2COOH���ж���ͬ���칹�壮���У�
���ܷ���������Ӧ������������Ʒ�Ӧ��������������ȩ�����ǻ��������ܷ�����ȥ��Ӧ����ṹ��ʽΪ  ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
����������������ˮ�⣬������ˮ�������Է���������ͬ������������γɵ������ṹ��ʽΪ�� ������Ϊ���������
��������������� �����������������
�����������������
�ʴ�Ϊ�� ��
�� ��
��
���� ���⿼���л����ƶϣ��漰±����������ȩ�������������ת��������ȷ��A�ķ���ʽ�ǹؼ�����Ҫѧ�������������չ����ŵ�������ת�����Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 235 | B�� | 143 | C�� | 92 | D�� | 51 |
| A�� | pH=7����Һ | |
| B�� | c��H+��=c��OH-��=10-6mol/L��Һ | |
| C�� | c��H+���T10-7mol/L | |
| D�� | �����ǡ����ȫ��Ӧ�������ε���Һ |
| A�� | �ô���ȥ��ˮ����CaCO3+2H+�TCa2++H2O+CO2�� | |
| B�� | ��ȩ������Cu��OH��2��Һ��Ӧ��NaOH+CH3CHO+2Cu��OH��2$\stackrel{��}{��}$CH3COONa+Cu2O��+3H2O | |
| C�� | ʵ������Һ��ͱ��ڴ������������屽�� +Br2$\stackrel{FeBr_{3}}{��}$ +Br2$\stackrel{FeBr_{3}}{��}$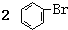 | |
| D�� | ��CO2ͨ�뱽������Һ��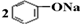 +CO2+H2O�� +CO2+H2O��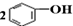 +Na2CO3 +Na2CO3 |
| A�� | ԭ��������24 | |
| B�� | ԭ�Ӱ뾶����ԭ�ӵ�ԭ�Ӱ뾶�� | |
| C�� | �����������SeO3�������������� | |
| D�� | ��̬�⻯�ﻯѧʽ��H2Se����ԭ�Ա�HClǿ |
| ������ | K+ Cu2+ Fe3+ Al3+ Fe2+ |
| ������ | Cl-CO32- NO3- SO42- SiO32- |
���ò�˿պȡ������Һ���ڻ��������գ�����ɫ���棨����ɫ�ܲ����۲죩��
��ȡ������Һ������KSCN��Һ�����Ա仯��
����ȡ��Һ�����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ����ʱ��Һ��Ȼ���壬����Һ������������䣮
���� ��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɣ�
���ƶϣ�
��1���ɢ��жϣ���Һ��һ�������е���������K+��Fe3+��д���ӷ��ţ���
��2�����м�����������������ɫ��������ӷ���ʽ��3Fe2++NO3-+4H+�T3Fe3++NO��+2H2O��
��3�����������ú���ɫ����ͨ��ˮ�У��������ɫ���������Ļ�ѧ����ʽΪ3NO2+H2O�T2HNO3+NO
��4����ͬѧ����ȷ��ԭ��Һ��������������Fe2+��Cu2+������������Cl-��NO3-��SO42-����д���ӷ��ţ�
��5����ȡ100mLԭ��Һ������������NaOH��Һ����ַ�Ӧ����ˣ�ϴ�ӣ����������أ��õ��Ĺ�������Ϊ1.6 g��
 ��
��