��Ŀ����
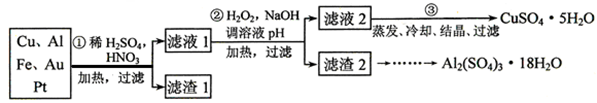
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�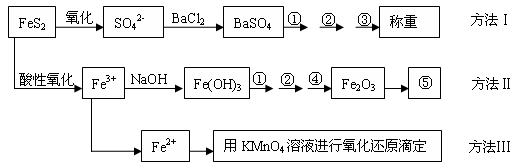
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
(5)��ȡ��ʯ����1.60 g, ��������������Ƶ�BaSO4������Ϊ4.66 g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������___________��
��1������ ��1�֣� ϴ�� ��1�֣� ���� ��1�֣� �������ƾ��� ��1�֣� ��ƽ ��1�֣�
��2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ ��2�֣�
��3��A��B��D ��3�֣�
��4����2�֣����������ܵ�ԭ��д������һ�֣�����2�֣�
�� Fe(OH)3���������������������
�ڹ���ϴ��ʱδ��ֽ�����������ϴȥ
�� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3
��5��75.0% ��3�֣�
���������������1������Һ�еõ������Ĺ����辭�����ˡ�ϴ�ӡ������������ڢܲ�Ϊ����ʹ���������ֽ�Ϊ��������Ȼ�����������������ȷ��FeS2����������ʹ�õ������ֱ�Ϊ�������ƾ��ƺ���ƽ����2��ȡ�ϲ���Һ�μ�BaCl2��Һ�����ް�ɫ�������ɣ�˵��SO42-������ȫ����3��A��������ҺҪ������ƿ������B����Ͳ�Ķ���ֻ�ܾ�ȷ��ʮ��λ������D���ζ���Ҫ�ô�װҺ��ϴ������4�������Ĺ��������ߣ����½��ƫ�ߣ��ʿ��ܵ�ԭ���У��� Fe(OH)3��������������������� �ڹ���ϴ��ʱδ��ֽ�����������ϴȥ �� Fe(OH)3���ղ���֣�δ��ȫת��ΪFe2O3��
��5��n(FeS2)=n(SO42-)/2=2n(BaSO4)/2=4.66��233��2=0.01mol
�ÿ�ʯ��FeS2������������0.01��120��1.60=0.75
���㣺���鶨��ʵ��Ļ�����������������������й����⡣

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
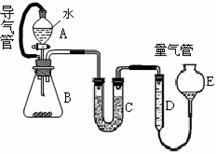
ȫ�Ų��Ծ�ϵ�д�Ϊ̽����ҵ���ϵ������ã�ij��ѧ��ȤС�������������ͼʵ�鷽�����ú�����������ͭ�ĺϽ���ȡ�Ȼ������̷�����(FeSO4��7H2O)�͵������塣 
��ش�
��1��������о�����е�ʵ������� ���ò����г��õ��ձ��Ͳ������⣬�������õ��IJ��������� ��
��2���Լ�X��
��3��������з�����Ӧ�����ӷ���ʽ�ǣ�_______________________________________
��4�����в����ʱ����С����������ͼ��ʾװ�ü��Լ����Ƶõ�CO2����ͨ����ҺA�С�һ��ʱ��۲쵽�ձ��в����İ�ɫ�������٣�Ϊ�˱������C���٣��Ľ��Ĵ�ʩ����װ�â�֮������һ�� ________________________________________��
��5����ҺD�и����ӵ�Ũ���ɴ�С��˳��Ϊ��__________________________________
��6����ҵ����X��F�Ƶ�CuSO4��������ʹ�õ���ǡ�����Լ�������_____��_________��
| A��ŨH2SO4 | B��Fe2O3 | C��HNO3 | D��O2 E.H2O2 |
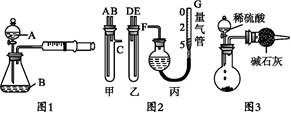
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȡ�����д���пհף�
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȡ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��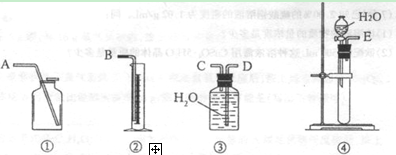
��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������±���
| | �������� | ����/g |
| ��ƿ��ˮ������ | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |
�ټ���CaC2����������ʱ����������6�ζ�����ԭ���ǣ� ��
�ڴ�������CaC2����������Ϊ ��(����2λ��Ч����)
��3�������ַ�������ȡһ��������������1.60g���������������£�

�ٲ������������ ��
����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ��� ���ƫ����ƫС�����䡱����
��һ��(4��)�����е����������ʣ�д����ȥ��Щ���ʵ��Լ���
��1��MgO (Al2O3) ��2��Cl2(HCl)
��3��FeCl3(FeCl2) ��4��NaHCO3��Һ(Na2CO3)
������(6��)��ˮ�к��д������Ȼ�þ���Ӻ�ˮ����ȡþ��������������ͼ��ʾ��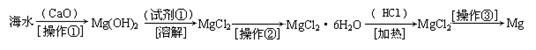
�ش��������⣺
д���ں�ˮ�м�������������������þ�Ļ�ѧ����ʽ ��
��������Ҫ��ָ ���Լ��ٿ�ѡ�� ��
��������ָ �������������տɵý���þ��
��������8�֣�ʵ��������480ml 0��1mol��L-1��Na2CO3��Һ���ش��������⣺
��1��Ӧ��������ƽ��ȡʮˮ̼���ƾ��� g��
��2����ͼ��ʾ������������Һ�϶�����Ҫ���� (�����)����ʵ�����貣������E���Ϊ mL��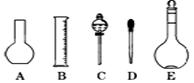
��3������ƿ�ϱ��У����¶ȡ���Ũ�ȡ�����������ѹǿ���ݿ̶��ߡ�����ʽ���ʽ�������е� ���������ַ��ţ�
��4�������������Ҫ�����ǣ�a����ƿ��b�ձ���c��ͷ�ιܡ�d������ƽ�������ڲ���������ʹ�õ�ǰ��˳���� ������д��ĸ��ÿ������ֻ��ѡ��һ�Σ�
��5���������ǻ�ѧʵ���г��õ�һ�ֲ������ߣ�����������Һ�Ĺ����в����������� ����;������д���֣�
��6����ʵ��ʱ���������������ʹ��Һ��Ũ��ƫ�͵��� ��
| A������ǰû�н�����ƿ�е�ˮ������ |
| B��̼����ʧȥ�˲��ֽᾧˮ�� |
| C��̼���ƾ��岻�������л����Ȼ��ƣ� |
| D������̼���ƾ���ʱ�����������⣻ |
����ʵ������д������
| A����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ���� |
| B���������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ��֧�ܿڴ� |
| C����Һ����ʱ����Һ©���²�Һ��Ӧ���¿ڷų����ϲ�Һ��Ӧ���Ͽڵ��� |
| D����ȡ����ʱ����ѡ��ȡ���ܽ���������Ӧ����ԭ�ܼ�����ԭ�ܼ��������� |