��Ŀ����
���к�CaO���ʵ�CaC2������ij�о���ѧϰС���ͬѧ�����������ַ����ⶨCaC2�����Ĵ��ȡ�����д���пհף�
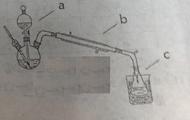
��1����һ�ַ����������ͼ��ѡ���ʵ���װ�ã����һ��ʵ�飬�ⶨCaC2�����Ĵ��ȡ�
��ѡ��װ�õ�����˳��Ϊ������ӿڵ���ĸ���� ��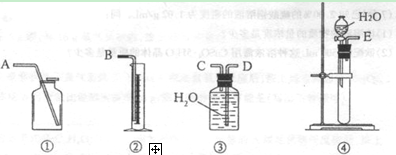
��2���ڶ��ַ���������������ˮ����ƿ�з�Ӧǰ�������ı仯���ⶨCaC2�������������ȳ�ȡ����1.50g����������ƿ��ˮ������Ϊ195.00g���ٽ�����������ƿ�У���Ӧ������ÿ����ͬʱ���õ��������±���
| | �������� | ����/g |
| ��ƿ��ˮ������ | ��1�� | 196.30 |
| ��2�� | 196.15 | |
| ��3�� | 196.05 | |
| ��4�� | 196.00 | |
| ��5�� | 196.00 |
�ټ���CaC2����������ʱ����������6�ζ�����ԭ���ǣ� ��
�ڴ�������CaC2����������Ϊ ��(����2λ��Ч����)
��3�������ַ�������ȡһ��������������1.60g���������������£�

�ٲ������������ ��
����ת����Һʱ������Һת�Ʋ���ȫ����CaC2���������IJⶨ��� ���ƫ����ƫС�����䡱����
��E��C��D�� B ����ƿ�������Ѵ���أ�82�G���Ǣ������ᾧ����ƫ��
��������������Ÿ���Ϊͨ���������ɵ���Ȳ���������ȷ��CaC2�����Ĵ��ȡ�����ΪE C
C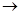 D
D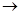 B
B
�� CaC2��2H2O Ca(OH)2��C2H2��
Ca(OH)2��C2H2��
64 26
x (195��1.5��196) =0.5g
��֮�ã�x=1.23g
��������CaC2������������
��3������Һת�Ʋ���ȫ�����õ����������ƫ������CaC2���������IJⶨ���ƫ��
���㣺����������ԭ��Ӧ�Լ�������й�֪ʶ��

 ��У����ϵ�д�
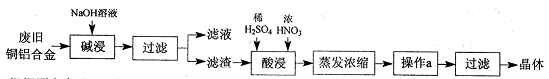
��У����ϵ�д�̼��������ҹ���Ҫ�ĵ���Ʒ��֮һ���������������������ӷ���ʧ��Ϊ�˼�����������ȷ�����ʩ����������ⶨ�京������
��ijѧ�������һ���Բⶨ������̼������Ӳⶨ�������ķ���������Ʒ����Բ����ƿ�У�

��1����ѡ���Ҫ��װ�ã���������������˳��Ϊ ��
��2����Һ©���е�Һ�����ʺϵ��� ��
| A��ϡ���� | B��ϡ���� | C��Ũ���� | D���������� |
����������гɷ��ǣ�NH4��2SO4��������ü�ȩ���ⶨ����������ȩ���ǻ��ڼ�ȩ��һ������������ã������൱�����ᣬ��ӦΪ2��NH4��2SO4+6HCHO����CH2��6N4 +2H2SO4 + 6H2O,���ɵ��������������Ʊ���Һ�ζ����Ӷ��ⶨ���ĺ������������£�
��1���ò�������ȡ���壨NH4��2SO4��Ʒ0��6g���ձ��У�����Լ30mL����ˮ�ܽ⣬�������100mL��Һ���� �����ʽ����ʽ�����ζ���ȷȡ��20��00mL����Һ����ƿ�У�����18%���Լ�ȩ��Һ5mL������5min����1~2�� ָʾ������֪�ζ��յ��pHԼΪ8��8������Ũ��Ϊ0��08mol/L�������Ʊ���Һ�ζ����������±���
| �ζ����� | �ζ�ǰ������mL�� | �ζ��������mL�� |
| 1 | 1��20 | 16��21 |
| 2 | 3��00 | 18��90 |
| 3 | 4��50 | 19��49 |
��ζ��յ�ʱ������Ϊ ���ɴ˿ɼ��������Ʒ�еĵ�����������Ϊ ��
��2���ڵζ�ʵ��������ֵζ��õļ�ʽ�ζ��ܲ��������ڳ��������ݣ��ζ���ʼʱ�����ݣ����ʵ��ⶨ�ĺ�������ʵ��ֵ ���ƫ��ƫС������Ӱ�족����
������ⶨ̼������еĺ�����ʱ��ʹ�ü�ȩ���Ƿ���� ����ǡ����������� ��
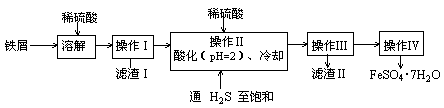
�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�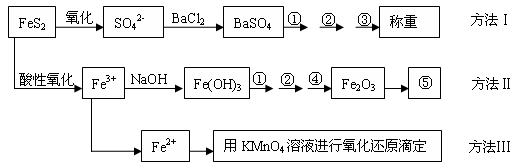
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
(5)��ȡ��ʯ����1.60 g, ��������������Ƶ�BaSO4������Ϊ4.66 g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������___________��
Ϊ�ᴿ�������ʣ�������Ϊ���ʣ�����ѡ�Լ�����������ȷ����
| ѡ�� | ���� | �����Լ� | ���� |
| A | �屽���壩 | CCl4 | ��Һ |
| B | NH3��H2O�� | Ũ���� | ϴ�� |
| C | ���飨��ϩ�� | ��ˮ | ϴ�� |
| D | CO2��SO2�� | Na2CO3������Һ | ϴ�� |

 ���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��
���˴�Ӧʹ��______�����Ũ�����ᡱ��ϡ���ᡱ����ԭ���� ______����ȡSO2��װ�ã����ѡ����ͼ�е�______��

 ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol
ͨ����Na2S2O3����Һ���Լ��ԣ��ζ���Ӧ�������ɵ�I2���������ĺ������ζ��������õ��IJ���������_______��ʵ����ȷ����0.1200g������Ʒ���ζ�������0.2000mol ��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ ��
��Na2S2O3��Һ27.60mL���������Ʒ��������������Ϊ �� CH3COOC2H5+H20
CH3COOC2H5+H20