��Ŀ����
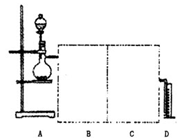
ijУ��ѧС���ͬѧ��һ����������·������õ���Cu��Al��Fe������Au��Pt�Ƚ����Ļ���������������Ʊ�����ͭ������������ķ�����
�ش��������⣺
��1���ڢٲ��μӷ�Ӧ�Ľ����� �֡�
��2���ڢڲ�����H2O2����Ϊ��Һ1�к��� ���ӡ�ʹ��H2O2���ŵ��� ��
��3�� �õڢ۲�����CuSO4��5H2O�Ʊ���ˮ����ͭ�ķ����ǣ� ��
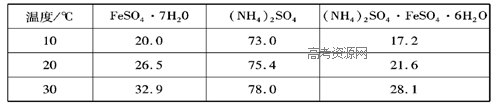
��4���������ѧС���ͬѧ���������2��ȡAl2(SO4)3��18H2O ��ʵ�鲽�裺
��ȡ����2������������ ����ַ�Ӧ����ˣ�
��ȡ��Һ������������ ����д�Լ��Ļ�ѧʽ����Ȼ�� ����д����ʵ����������ƣ���
��3��������ϡ�����ܽ⣻
��4����� ����д����ʵ����������ƣ������Al2(SO4)3��18H2O���塣
��1���ڢ۲�����CuSO4��5H2O���п���������Na2SO4��Ϊ�����������ⶨCuSO4��5H2O�Ĵ��ȣ�ѡ��BaCl2(aq)��������Ҫ���Լ������г�����ⶨ�������� ��
��1��3 ��1�֣�
��2�� Fe2+ ��1�֣��� ���������ʣ���ԭ������ˮ���Ի�������Ⱦ��1�֣�
��3�������¶ȼ��Ȼ����գ�1�֣�
��4��������������Һ��1�֣� �ڶ�����̼���������ᣬ����ϴ�ӣ�1�֡�2��û��ϴ�ӿ�1�֣���4��������������ȴ�ᾧ������ϴ�� ��1�֡�3��
��5��m����������m�����غ��BaSO4�� ��1�֡�2��������ȷ���֣�
�������������39��ϡ���ᡢŨ�����������ȣ�Cu��Al��Fe������Ӧ����Cu2+��Al3+��Fe2+����������1�ijɷ���Pt��Au����Һ1�е�������Cu2+��Al3+��Fe2+���ڢٲ�Cu���ᷴӦ�����ӷ���ʽΪ��Cu+4H++2NO3- Cu2++2NO2��+2H2O ��3Cu+8H++2NO3-
Cu2++2NO2��+2H2O ��3Cu+8H++2NO3- 3Cu2++2NO��+4H2O��Au��Pt�� 40���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+���������������������������ʣ��Ի�������Ⱦ��41����
3Cu2++2NO��+4H2O��Au��Pt�� 40���ڢڲ���H2O2�������ǽ�Fe2+����ΪFe3+���������������������������ʣ��Ի�������Ⱦ��41����
�۲�����ˮ����ͭ�Ʊ�����ͭ�ķ���Ӧ���������м�����ˮ��42����1���������м�NaOH��Al��OH��3��Ӧ����NaAlO2��������Һ�м�H2SO4����Al2��SO4��3����������ȴ���ᾧ�����˿ɵ����������壻����ԭ�����ýǶȿ��Ƿ����Ҹ���������Ϊ���ӵ�NaOH���Ʊ���Al2��SO4��3��ԭ�����û�й�ϵ�����ԭ���˷ѣ���2��������̼���������ᣬ����ϴ�ӣ���4�����������������ȴ�ᾧ������ϴ�� �����Al2(SO4)3��18H2O���塣 43���������m����������m�����غ��BaSO4����������
���㣺���⿼�����ӷ���ķ�����ʵ����ƣ��Լ�ѡ���к͵ζ��ļ�������ķ������������ʵ����������ǽ���ؼ�����Ŀ�Ѷ��еȣ�

�����Ƿ�����������FeS2���������ַ������������IJ�������ͼ���£�
��ش��������⣺
(1)����ͼ�в����١��ڡ��۷ֱ�ָ���Ǣ�_________����__________����________��
�����ܡ����õ�����Ҫ�����ǣ���__________����__________(ÿ����1-2������)��
(2)�ж���Һ��SO42-�����ѳ�����ȫ�ķ�����_________________________________��
(3)ijͬѧ�÷�����ⶨ������FeԪ�صĺ�����ȷ��ȡһ�����Ŀ�ʯ�������������ܽ⡢Ԥ������
| A���ô��п̶ȵ��ձ����Ƴ�100 mL������Һ�� | B������Ͳ��ȡ25.00 mL������Һ�� | C����������ƿ�С� | D��������ˮϴ�ӵζ��ܺ�װ��KMnO4����Һ���øñ���Һ�ζ�����������(E)����Һ��ɵ��Ϻ�ɫʱ��ֹͣ�ζ�����30���ڲ���ɫ��(F)��ȡ������ζ��������ĵ�KMnO4����Һ��������������е�FeԪ�غ�������ָ����ʵ������д����������ı��________________________�� |
(5)��ȡ��ʯ����1.60 g, ��������������Ƶ�BaSO4������Ϊ4.66 g�������ʯ�е���Ԫ��ȫ��������FeS2����ÿ�ʯ��FeS2������������___________��
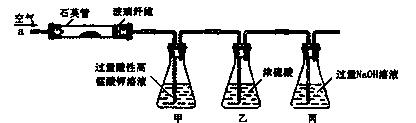
�Ͼ���Ļ������ü������ڽ�Լ��Դ���������ڱ���������ij�о�С��ͬѧ�ԷϾ�п�̸ɵ��Ϊԭ�ϣ����Ͼɵ�غ�п����ת����ZnSO4��7H2O�����̲���ת���ɴ��Ƚϸߵ�MnO2����NH4Cl��ҺӦ���ڻ��������У�ʵ���������£�
��1�������������õļ�������Ӧѡ ��ѡ�����������������
��2������ҺA�����ĵ�һ���Ǽ��백ˮ����pHΪ9��ʹ���е�Fe3+��Zn2+��������д����ˮ��Fe3+��Ӧ�����ӷ���ʽ ��
��3����������Ϊ�˳�ȥ��Һ�е�Zn2+����֪25��ʱ��
| NH3��H2O��Kb | Zn2+��ȫ������pH | Zn(OH)2���ڼ��pH |
| 1.8��10��5 | 8.9 | ��11 |
���ϱ����ݷ���Ӧ������ҺpH���Ϊ ������ţ���
a��9 b��10 c��11
��4�� MnO2����������Ҫ���裺
����1����3%H2O2��6.0mol/L��H2SO4�Ļ��Һ����MnO2�ܽ⣬���ȳ�ȥ����H2O2����MnSO4��Һ��������Fe3+������Ӧ����MnSO4�����ӷ���ʽΪ ��
����2����ȴ�����£��μ�10%��ˮ����pHΪ6��ʹFe3+������ȫ���ټӻ���̿���裬���ˡ��ӻ���̿�������� ��
����3������Һ�еμ�0.5mol/L��Na2CO3��Һ������pH��7���˳�������ϴ�ӡ�����������ں�ɫ������MnO2�����չ����з�Ӧ�Ļ�ѧ����ʽΪ ��
��5�� ������֪����MnO2���ܽ�������������������ݣ�Ȼ����ȡMnCO3���塣
���������������Һ��Ũ�Ⱦ�Ϊ5mol/L�������Ⱥ���ѽ���ʱ���£������¶ȶ�MnCO3���ʵ�Ӱ����ͼ4����ͼ�������������ѽ����¶ȶ��� �����ң�
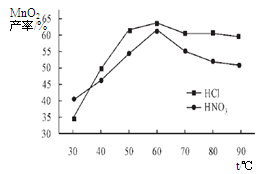
��������¶ȡ���ѽ���ʱ����������£����Ũ�ȶ�MnCO3���ʵ�Ӱ����ͼ5����ͼ������������Ũ��Ӧѡ�� mol/L���ҡ�

�����й�ʵ��ԭ���������ͽ��۶���ȷ����
| A����FeCl3��Һ�еμӹ�����ˮ������ȡFe(OH)3���� |
| B��ȡ������ҺX�������м����������ư�ˮ���ټӼ���KSCN��Һ����Һ��죬˵��X��Һ��һ������Fe2�� |
| C�����������������ӵĻ����Һ�м�������NaOH��Һ�������ú��Һ���ɳ�ȥ������������ |
D����֪I3�� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� I2��I������ʢ��KI3��Һ���Թ��м�������CCl4�����ú�CCl4������ɫ��˵��KI3��CCl4�е��ܽ�ȱ���ˮ�еĴ� |
���������������ص������У���ȷ����
| A������������ʱ��Ѹ�ٽ��Ҵ�ע��Ũ������ |
| B����ʯ������װ���У����¶ȼ�ˮ�������Һ������ |
| C���ò�˿պȡ����KCl��Һ���ڻ��������գ�ֱ�ӹ۲������ɫ������K+�Ĵ��� |
| D����Һ����ʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |