��Ŀ����
3����NAΪ�����ӵ�������ֵ��������˵����ȷ���ǣ�������| A�� | ��⾫��ͭʱ����������������6.4 g�����·��ת�Ƶ�����Ϊ0.2NA | |
| B�� | ��NH4Al��SO4��2ϡ��Һ����μ������Ba��OH��2��Һ�����ӷ���ʽΪ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+AlO2-+2H2O+NH3•H2O | |
| C�� | �����£���ˮ�������H+Ũ��Ϊ10-13mol•L-1����Һ�У�Fe2+��Cl-��Na+��NO3-���ܴ������� | |
| D�� | �����ǣ�C6H12O6����Һ�У�SO42-��MnO4-��K+��H+���Դ������� |
���� A����ͭ�к��л�ԭ��ǿ��ͭ����������п�������������ȷŵ磻
B����дһ�����������ӷ���ʽʱ�������ٵ����ʵ����ʵ���Ϊ1mol�����������ĵ���������д��
C����ˮ�������H+Ũ��Ϊ10-13mol•L-1����Һ��ˮ�ĵ��뱻���ƣ�����������ҺҲ�����Ǽ���Һ��
D���������Ƕ��ǻ���ȩ����ȩ������ǿ��ԭ�ԣ�
��� �⣺A����⾫��ͭʱ��������ͭ�к��л����Խ�ǿ�������ȵ����ʣ����ʱ����п�����ȷŵ磬����Ħ������С��ͭ��64g/mol��п��Ħ����������ͭ��Ħ��������������������6.4g��ת�Ƶĵ��ӵ����ʵ�����һ��Ϊ0.2mol����A����
B����дһ�����������ӷ���ʽʱ�������ٵ�NH4Al��SO4��2�����ʵ���Ϊ1mol����1molNH4+����1molOH-��1molAl3+����4molOH-��2molSO42-����2molBa2+�������ӷ���ʽΪ��NH4++Al3++2SO42-+2Ba2++5OH-=2BaSO4��+AlO2-+2H2O+NH3•H2O����B��ȷ��
C����ˮ�������H+Ũ��Ϊ10-13mol•L-1����Һ��ˮ�ĵ��뱻���ƣ�����������ҺҲ�����Ǽ���Һ����������Һʱ���д����������ӣ���Fe2+��H+����NO3-���ܴ������棻��Ϊ����Һ������ڴ���OH-������Fe2+���ܹ��棬��C����
D���������Ƕ��ǻ���ȩ����ȩ������ǿ��ԭ�ԣ��ܱ�MnO4-���������ܹ��棬��D����
��ѡB��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

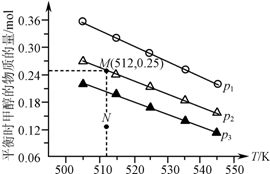 ��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������
��1.0L�����ܱ�������Ͷ��1mol CO2��2.75mol H2������Ӧ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g����ʵ���ò�ͬ�¶ȼ�ѹǿ�£�ƽ��ʱ�״������ʵ����仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | �÷�Ӧ������ӦΪ���ȷ�Ӧ | |
| B�� | ѹǿ��С��ϵΪp1��p2��p3 | |
| C�� | M���Ӧ��ƽ�ⳣ��K��ֵԼΪ1.04��10-2 | |
| D�� | ��p2��512 Kʱ��ͼ��N��v��������v���棩 |
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ��������Ƿ�ˮ������������ | �������Һ�м���ϡH2SO4�����ȼ����ӣ���ȴ��ˮ��Һ��NaOH��Һ�кͣ�Ȼ���ټ�������Cu��OH��2��Һ�����ȣ��۲��Ƿ��к�ɫ�������� |
| B | ����Fe��NO3��2�����Ƿ����������� | ��Fe��NO3��2��Ʒ����ϡ����μ�KSCN��Һ���۲���Һ�Ƿ��� |
| C | ��֤Br2��������ǿ��I2 | ��������ˮ����KI��Һ�У��ټ���CCl4�������ã��ɹ۲쵽�²�Һ�����ɫ |
| D | ��֤Fe��OH��3���ܽ��С��Mg��OH��2 | ��FeCl3��Һ����Mg��OH��2����Һ�У����ɹ۲쵽�����ɰ�ɫ��Ϊ���ɫ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A | B | C | D | |
| �� Ʒ |  |  |  |  |
| ��Ҫ�ɷ� | CO2 | Fe2O3 | NaHCO3 | CH3COOH |
| ��; | ������� | ����ɫͿ�� | ������ | ����ζ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
��Cl2���к�ǿ�������ԣ��ڻ�ѧ��Ӧ��ֻ����������
��ŨH2SO4��ǿ�����ԣ�����������Cu�������ҷ�Ӧ
�ۻ��Ϸ�Ӧ��Ϊ������ԭ��Ӧ
��Cl2��SO2����ʹƷ����Һ��ɫ��˵�����߾���������
��2Na2O2+2H2O=4NaOH+O2����Cl2+H2O=HCl+HClO ��������Ӧ��ˮ������ԭ����
| A�� | �٢ڲ���ȷ���ۢܢ���ȷ | B�� | �٢ڢۢܢݾ�����ȷ | ||
| C�� | �٢���ȷ���ۢܢݲ���ȷ | D�� | �٢ڢۢ���ȷ |
| A�� | Na2A��Һ�У�c��Na+����c��A2-����c ��HA-����c��H2A�� | |
| B�� | NaHA��Һ�У�c��Na+��=c��A2-��+c��HA-�� | |
| C�� | Na2A��Һ�У�c��OH-��=c��H+��+c��HA-��+2c��H2A�� | |
| D�� | ������0.1mol/L��H2A��ҺpH����1 |
| A�� | ���������������̼ԭ�� | |
| B�� | �����������һ���һ���һ���Ȼ����� | |
| C�� | ������ӵĹ��������Ȼ� | |
| D�� | ��������к���һ���ǻ� |
 ��
��