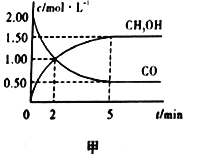
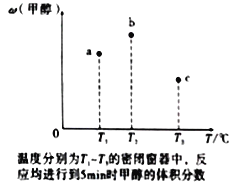
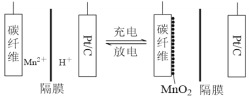
��Ŀ����
����Ŀ��������������Һ�е�ָ��������100 mL 3.9 mol/L Ba(NO3)2��Һ����NO3�����ʵ���Ũ����ͬ����
A.390 mL 0.1 mol/L MgCl2��Һ�е�Cl��
B.200 g Ũ��Ϊ26%���ܶ�Ϊ1.2g/mLNaOH��Һ�е�OH��
C.50 mL 7.8 mol/L Al2(SO4)3��Һ�е�SO42��
D.200 mL 3.9 mol/L CaCl2��Һ�е�Ca2��
���𰸡�B
��������
3.9 mol/L Ba(NO3)2��Һ����NO3�����ʵ���Ũ��Ϊ3.9 mol/L��2=7.8 mol/L��
A. 390 mL 0.1 mol/L MgCl2��Һ�е�c(Cl��)=0.1 mol/L��2=0.2 mol/L���ʲ�ѡA��
B. 200 g Ũ��Ϊ26%���ܶ�Ϊ1.2g/mLNaOH��Һ��c(OH��)= c(NaOH)=![]() 7.8 mol/L����ѡB��
7.8 mol/L����ѡB��
C. 50 mL 7.8 mol/L Al2(SO4)3��Һ��c(SO42��)= 7.8 mol/L��3=23.4 mol/L���ʲ�ѡC��
D. 200 mL 3.9 mol/L CaCl2��Һ�е�c(Ca2��)= 3.9 mol/L��1=3.9 mol/L���ʲ�ѡD��

 Ŀ�����ϵ�д�
Ŀ�����ϵ�д�����Ŀ���ס��ҡ�����ȡ300mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһþ���Ͻ��ĩ��������ʵ�飬�й������б����£�
ʵ����� | �� | �� | �� |
�Ͻ�����/mg | 510 | 765 | 918 |
(��״��)�������/mL | 560 | 672 | 672 |
��1����������ʵ���Ũ���Ƕ���___��
��2���Ͻ���þ���������������Ƕ���___��___��