��Ŀ����
����Ŀ����˧��ʿͬ�������ݴ�ѧ��������У����ϵEnrique Iglesia�����ڽ������������������ȩ���Ϸ�Ӧ��������ĺ����о�ȡ���½�չ����ͼ���ʻ�������������Ϻͽ�������;����
�ش��������⣺
(1)��̬Oԭ�Ӻ�����____________�ֲ�ͬ�˶�״̬�ĵ��ӣ���OԪ��ͬ���ڵ�һ�����ܴ���O������Ԫ����___________(��Ԫ�ط���)��
(2) HCHO��H2O����������ת����HCHO���ӵĿռ乹����________�����ۼ�ȩ�Ľṹ��ͼ��ʾ�����ۼ�ȩ��Cԭ�ӵ��ӻ���ʽΪ_____________��
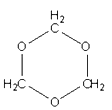
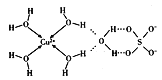
(3)H2O���γɶ�����λ�����������Աͨ��X�����Ʋ���мȺ�����λ�����ֺ���������ṹ��ͼ��ʾ�������Ļ�ѧʽ����λ���������ʽ�ɱ�ʾΪ____��1mol����������������ĿΪ_________NA��
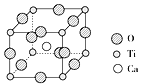
(4)TiO2������ת���Ĵ�����CaTiO3����ȡTiO2��һ��ԭ�ϣ��侧���ṹ��ͼ��ʾ����CaTiO3�����У���ij�������Ӿ����������ȵ����������ӹ���________������û������ʽ��ΪM���ܶ�Ϊa g/cm3�������ӵ�����ΪNA�������и�������������֮�����̾���Ϊ________cm��
���𰸡�8 N��F ƽ�������� sp3 ��Cu(H2O)4��SO4��H2O 18 6 ![]() ��
��![]()
��������
��1����̬O�ĺ�������Ų�Ϊ1s22s22p4��ÿ�����Ӷ����Լ����˶�״̬��ͬ��������Ԫ�أ�����ԭ����������һ�����ܳ���������ƣ����ڢ�A��͵ڢ�A�巴����
��2���ü�ȩ�еijɼ�������̼ԭ�ӵ��ӻ����ͣ������ӻ������������ռ�ṹ��
��3����������ˮ������ͭ������ͼ����ʾ��ʾ������������H2O�ں���������Cu2+��H2O֮����ڵ���λ��ҲΪ������SO42-��S��O֮�����һ��������
��4�����ݾ����ṹͼ��֪��ÿ������Χ�������������������Ķ��㣬����Ϊ���������ߵı߳�������������6������û������ʽ��ΪM���ܶ�Ϊa g/cm3�������ӵ�����ΪNA����������Ϊ![]() cm3���߳�Ϊ
cm3���߳�Ϊ![]() ���Դ˿ɼ����������������֮�����̾��롣
���Դ˿ɼ����������������֮�����̾��롣
(1)��̬O�ĺ�������Ų�Ϊ1s22s22p4��ÿ�����Ӷ����Լ����˶�״̬�����Ի�̬Oԭ�Ӻ�����8�ֲ�ͬ�˶�״̬�ĵ��ӣ�
ͬ��������Ԫ�أ�����ԭ����������һ�����ܳ���������ƣ����ڢ�A��͵ڢ�A�巴��������OԪ��ͬ���ڵ�һ�����ܴ���O������Ԫ����N��F��
�ʴ�Ϊ��8��N��F��
(2)��ȩ�����к���̼��˫��������3����������̼ԭ�ӹ�����ӻ�����Ϊsp2�ӻ������ȩ��̼ԭ�Ӳ�ȡsp2�ӻ�������ӵĿռ乹��Ϊƽ�������Σ�
�������ۼ�ȩ�Ľṹ������C�ļ۵��Ӷ���ΪVP=BP+LP=4+![]() =4����Cԭ�ӵ��ӻ���ʽΪsp3��
=4����Cԭ�ӵ��ӻ���ʽΪsp3��
�ʴ�Ϊ��ƽ��������;sp3��
(3)��������ˮ������ͭ������ͼ����ʾ��Cu2+��Χ��4������H2O�����Ļ�ѧʽ����λ���������ʽ�ɱ�ʾΪ����Cu(H2O)4��SO4��H2O��
Cu2+��H2O֮����ڵ���λ��ҲΪ������SO42-��S��O֮�����һ����������1mol����������������ĿΪ3��4+2+4=18mol��
�ʴ�Ϊ����Cu(H2O)4��SO4��H2O��18��
(4)���ݾ����ṹͼ��֪��ÿ������Χ�������������������Ķ��㣬����Ϊ���������ߵı߳�������������6����
��û������ʽ��ΪM���ܶ�Ϊag/cm3�������ӵ�����ΪNA����������Ϊ![]() cm3���߳�Ϊ
cm3���߳�Ϊ![]() �����������������֮�����̾���Ϊ
�����������������֮�����̾���Ϊ ![]() ��
��![]() ��
��
�ʴ�Ϊ��6; ![]() ����
����![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
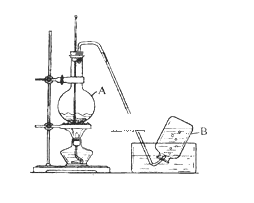
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ˮ����þ��MgSO4��7H2O����ӡȾ����ֽ��ҽҩ�ȹ�ҵ������Ҫ����;����þ������þ��������ɰ�ķ���������Ҫ�ɷ���MgCO3��������MgO��CaO��Fe2O3��FeO��MnO2��Al2O3��SiO2�����ʣ���ҵ������þ����ȡ��ˮ����þ�Ĺ���������ͼ��
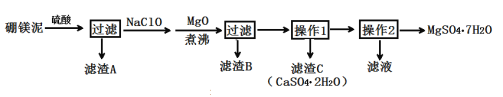
��֪����MnO2������ϡ���ᡣ
��CaSO4��MgSO4��7H2O�ڲ�ͬ�¶��µ��ܽ�ȣ�g���������±���ʾ��
�¶�/�� ���� | 10 | 30 | 40 | 50 | 60 |
CaSO4 | 0.19 | 0.21 | 0.21 | 0.21 | 0.19 |
MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 | ���� |
��1����ʼ�õ��������������Ϊ70%���ܶ�Ϊ1.61g/cm3�����������Һ�����ʵ���Ũ��Ϊ___��
��2������A�г�������CaSO4��2H2O�⣬����___��
��3������MgO������е�Ŀ����___��
��4��������B����Ҫ�ɷ�ΪAl(OH)3��Fe(OH)3�������NaClO����������ԭ��Ӧ�����ӷ���ʽΪ___��
��5�������в���1Ϊ����Ũ�������ȹ��ˣ��������ɵõ�CaSO4��2H2O���ַ�ֹ___��
��6����ȡMgSO4��7H2O�IJ���2Ϊ��___��___������ϴ�ӡ�
��7����֪��ʼ��þ����Ʒ������Ϊag����ȡ��ˮ����þ������Ϊbg���ݴ��ܼ������þ����þԪ�صĺ��������ܣ���д������ʽ�������ܣ���˵�����ɡ�___���ܻ��ܣ�������ʽ�������ɣ�Ϊ___��