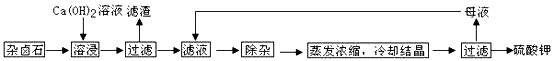
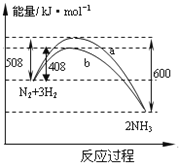
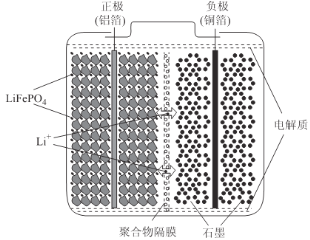
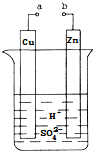
��Ŀ����
����Ŀ������ѧѡ��2����ѧ�뼼��������刺��������ʡ�����ըҩ��ɱ������䶳���ȡ���ҵ��ȡ����淋Ĺ����������£�
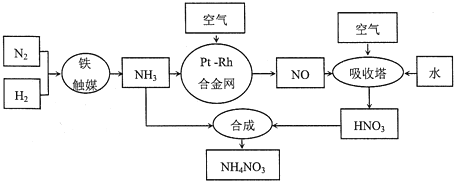
��1���ϳɰ����Ĺ�ҵ�豸����_______________���豸�������Ƚ�������Ŀ����____________���ϳɰ���ԭ��������������Ŀ����_______________��
��2���������з�Ӧ�Ļ�ѧ����ʽΪ_________________________�����������̿�������������Ҫ�����������ԭ����_______________��
��3����������Ĺ����г��������������������������ַ���������
����һ��������Һ���շ�
NO+NO2+2NaOH�T2NaNO2+H2O��2NO2+Na2CO3�TNaNO2+NaNO3+CO2
��������NH3��ԭ��
8NH3(g)+6NO2(g)�T7N2(g)+12H2O(g)��H=-2635kJ/mol(NOҲ�����Ƶķ�Ӧ)
���������������շ�
CH4(g)+2NO2�TCO2(g)+N2+2H2O(g)��H=+867kJ/mol(NOҲ�����Ƶķ�Ӧ)
�������ַ����У�����һ����Ҫȱ����_______________��
�������ͷ�������ȣ���������ȱ����_______________��
��4����ҵ��Ҳ����ͨ�����NO�Ʊ�NH4NO3���乤��ԭ����ͼ��ʾ��
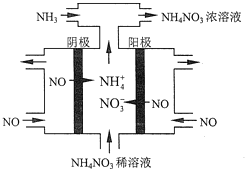
��ͼ��ͨ��NH3��Ŀ����_______________��
����ά�ֵ���ǿ��Ϊ3A�����2Сʱ�������Ͽ��Ƶ�NH4NO3���������Ϊ_____g��(��֪F=96500Cmol-l)(����2λ��Ч����)��
��5��ij���ʳ���NH3�Ʊ�NH4NO3����֪��NH3��NO�IJ�����94%��NO��HNO3�IJ�����89%������HNO3����NH3������ռ�ܺ�NH3����(�������������)�İٷֱ�Ϊ______________��
���𰸡���1���ϳ��� ����������ܣ���Լ��Դ ��ֹ�����ж�
��2��2NO+O2=2NO2��3NO2+H2O=2HNO3+NO(��4NO2+O2+2H2O=4HNO3) ��ʹNOѭ�����ã����ԭ��������
��3��������NO���ܱ����� ���ܸ�
��4����ʹ������ȫ��ת��ΪNH4NO3 �� 6.0 ��5��54.4%
��������
�����������1���ϳɰ��Ĺ�ҵ�豸�Ǻϳ������ϳɰ��ķ�Ӧ���ڷ��ȷ�Ӧ����Ӧ�����л�ų��������ȣ����Ƚ��������Գ���������ȣ���Լ��Դ��N2��H2�ϳ�NH3��������ԭ�����������������Է�ֹ�����ж���
��2�����������ж���������ˮ��Ӧ���������NO��ͨ�������NO�ܱ������е���������Ϊ��������2NO+O2=2NO2��������������ˮ��Ӧ��������3NO2+H2O=2HNO3+NO������ʹNOѭ�����ã�ȫ��ת��Ϊ���ᣬ��Ӧ�Ļ�ѧ����ʽΪ4NO+3O2+2H2O=4HNO3��
��3������һ����ȱ���ǵ�����NO���ܱ����գ�ֻ����NO2һ�𱻼�Һ���գ��������ͷ�������ȣ��ŵ��Ǽ���Ȱ��۸���ˣ���Լ�ɱ���ȱ���Ƿ������ķ�Ӧ��Ϊ+867kJmol-1�����ܽϸߣ�
��4�������NO�Ʊ�NH4NO3��������ӦΪNO-3e-+2H2O=NO3-+4H+��������ӦΪ��NO+5e-+6H+=NH4++H2O����������Ӧ�ɿ�����Ҫʹ��ʧ�����غ㣬����������NO3-�����ʵ�����������������NH4+�����ʵ������ܷ�Ӧ����ʽΪ��8NO+7H2O![]() 3NH4NO3+2HNO3����˲���NH3��ʹ������ȫ��ת��ΪNH4NO3��
3NH4NO3+2HNO3����˲���NH3��ʹ������ȫ��ת��ΪNH4NO3��
��ά�ֵ���ǿ��Ϊ3A�����2Сʱ��ʱ��Ϊ7200S��F=96500Cmol-1��Q=It=3.0C/s��7200S���������Ͽ��Ƶ�NH4NO3���������Ϊ![]() ��80g/mol=6.0g��
��80g/mol=6.0g��
��5����NH3��NO�IJ�����94%��NO��HNO3�IJ�����89%�����ݵ�ԭ���غ��֪��NH3��NO��HNO3����1mol�����ɵõ�����1mol��94%��89%=0.8366mol����HNO3+NH3�TNH4NO3����÷�Ӧ���ĵİ��������ʵ���Ϊ0.8366mol������������֮�ȵ������ʵ���֮�ȣ�����HNO3����ȥ��NH3������ռ�ܺ�NH3�����İٷ���Ϊ![]() ��100%=54.4%������HNO3����ȥ��NH3������ռ�ܺ�NH3������54.4%��
��100%=54.4%������HNO3����ȥ��NH3������ռ�ܺ�NH3������54.4%��