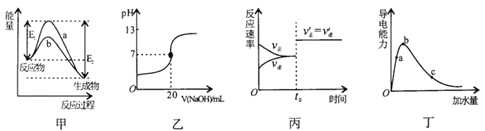
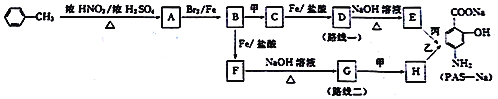
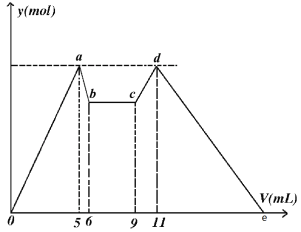
��Ŀ����
����Ŀ����ͭ��(��Ҫ�ɷ�CuFeS2)����ȡCu����Ҫԭ�ϡ�
��֪��2CuFeS2+4O2![]() Cu2S+3SO2+2FeO����
Cu2S+3SO2+2FeO����
����Cu2S��1200 ������¼�����Ӧ��2Cu2S+3O2==2Cu2O+2SO2 ����
2Cu2O+Cu2S==6Cu +SO2�� ������
�ٶ�������Ӧ����ȫ��������˵����ȷ����
A. ��Ӧ����CuFeS2������ԭ��
B. ȡ12.5g��ͭ����Ʒ�����ⶨ��3.60g���������CuFeS2��������һ��Ϊ82.8%
C. ��6molCuFeS2����6molCu����O2�����ʵ���Ϊ14.25mol
D. 6molCuFeS2��15.75molO2��Ӧ�������Ͽɵõ�ͭ�����ʵ���Ϊ3mol
���𰸡�D
��������A. ��Ӧ����ͭԪ�ػ��ϼ۽��ͣ���Ԫ�ػ��ϼ����ߣ�CuFeS2������ԭ����Ҳ����������A����B. ���ڲ���ȷ�������Ƿ�����Ԫ�أ����Բ��ܼ���CuFeS2�ĺ�����B����C. ���ݷ���ʽ��֪6molCuFeS2�μӷ�Ӧ������3molCu2S������2molCu2Sת��Ϊ2molCu2O��2molCu2O��1molCu2S��Ӧ����6molCu����������O2�����ʵ���Ϊ12mol+3mol��15mol��C����D. 6molCuFeS2��Ҫ12mol�����õ�3molCu2S��ʣ��3.75mol������������Cu2S�����ʵ�����![]() ���õ�2.5molCu2O����ʱʣ��Cu2S�����ʵ�����0.5mol����1molCu2O��Ӧ����3molͭ��D��ȷ����ѡD��
���õ�2.5molCu2O����ʱʣ��Cu2S�����ʵ�����0.5mol����1molCu2O��Ӧ����3molͭ��D��ȷ����ѡD��

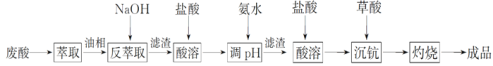
����Ŀ��KAl��SO4��2��12H2O����������һ�ָ��Σ�����ֽ�ȷ���Ӧ�ù㷺��ʵ�����У����÷������ޣ���Ҫ�ɷ�ΪAl������������Fe��Mg���ʣ��Ʊ������Ĺ�������ͼ��ʾ���ش��������⣺
![]()
��1��Ϊ�������������ʣ��Լ���Ӧѡ��___�����ţ���
A��HCl��Һ | B��H2SO4��Һ | C����ˮ | D��NaOH��Һ |
��2���������ܽ��������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________��
��3������B�Ļ�ѧʽΪ________����������������ˮ����Һ�������ԣ���ԭ����__________��
��4����֪��Kw=1.0��10-14��Al��OH��3![]() AlO2-+H++H2O K=2.0��10-13��Al(OH)3����NaOH��Һ��Ӧ��ƽ�ⳣ������_________��
AlO2-+H++H2O K=2.0��10-13��Al(OH)3����NaOH��Һ��Ӧ��ƽ�ⳣ������_________��