��Ŀ����
����Ŀ��ij�¶��£���һ�ܱ������г���һ����CO2���������������ۣ�������Ӧ��Fe(s)��CO2(g) ![]() FeO(s)��CO(g)�����CO2��COŨ����ʱ��ı仯��ͼ��ʾ��
FeO(s)��CO(g)�����CO2��COŨ����ʱ��ı仯��ͼ��ʾ��

��1��0��8 min��v(CO2)��__________mol��L��1��min��1��
��2���÷�Ӧ�ں��º��ݵ��ܱ������н��У���˵���÷�Ӧ�ﵽ��ѧƽ��״̬����_____
A����λʱ���� ��ÿ����1molCO2ͬʱ����1molCO
B�������������ѹǿ������ʱ��仯
C��������������ܶȲ�����ʱ��仯
D�������������ƽ����Է�������������ʱ��仯
��3��������¶��£���ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��__________
FeO(s)��CO(g)��ƽ�ⳣ��__________
��4�����д�ʩ�У��ܹ��ı�ƽ��ʱc(CO)/c(CO2)�ı�ֵ����________(�����)��
A���¶� B�����۵���(����) C��ѹǿ D��CO����
��5����֪����ӦFe(s)��CO2(g) ![]() FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2����ͬ�¶�ʱK1��K2��ֵ���±���
�¶�/K | K1 | K2 |
973 | 1.47 | 2.38 |
1 173 | 2.15 | 1.67 |
�ٷ�ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)Ϊ______(����ȡ����ȡ�)��Ӧ
FeO(s)��H2(g)Ϊ______(����ȡ����ȡ�)��Ӧ
�ڸ��ݱ������ݣ����㷴ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g) 973 K��K______��д������ʽ���ɣ����ؼ���������
CO(g)��H2O(g) 973 K��K______��д������ʽ���ɣ����ؼ���������
���𰸡�0.0625CD2A����1.47/2.38
��������
�⣺(1)0��8min��v(CO)=![]() =
=![]() =0.0625mol/(L��min)���ʴ�Ϊ��0.0625��
=0.0625mol/(L��min)���ʴ�Ϊ��0.0625��
(2)A����λʱ���� ��ÿ����1molCO2ͬʱ����1molCO������ʾ��������Ӧ���ʣ�����˵�����淴Ӧ�����Ƿ���ȣ���A����B����Ӧǰ����������ʵ������䣬�����������ѹǿʼ�ղ��䣬��B����C����ӦǰΪ������̼����Ӧ����һ����̼��������������ܶȲ�����ʱ��仯��˵��������̼��һ����̼�����ʵ���֮�Ȳ��䣬˵���ﵽ��ƽ��״̬����C��ȷ��D����Ӧǰ����������ʵ������䣬������������仯����������������ƽ����Է�������������ʱ��仯��˵���������䣬�ܹ�˵���ﵽƽ��״̬����D��ȷ����ѡCD��
(3)Fe(s)+CO2(g)FeO(s)+CO(g)��K1=![]() =
=![]() =2���ʴ�Ϊ��2��
=2���ʴ�Ϊ��2��
(4)A�������¶Ȼ��¶ȣ�ƽ��һ�������ƶ�����ñ�ֵһ�������仯����A��ȷ��B��Fe��Ϊ���壬���۵����ı䣬���ı�Ũ�ȣ�ƽ�ⲻ�ƶ�����ñ�ֵ�������仯����B����C����Ӧǰ����������ʵ������䣬�ı�ѹǿ��ƽ�ⲻ�ƶ�����ñ�ֵ�������仯����C����D���ı�CO����ƽ�ⷢ���ƶ������¶Ȳ��䣬K���䣬���Ըñ�ֵ���䣬��D��ȷ����ѡA��
(5)�����ݱ������ݣ���ӦFe(s)��H2O(g) ![]() FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���¶����ߣ�ƽ�ⳣ����С��˵��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
FeO(s)��H2(g)��ƽ�ⳣ��ΪK2���¶����ߣ�ƽ�ⳣ����С��˵��ƽ�������ƶ�������ӦΪ���ȷ�Ӧ���ʴ�Ϊ�����ȣ�
��i��Fe(s)+CO2(g)FeO(s)+CO(g)��K1=![]() ��ii��Fe(s)+H2O(g)FeO(s)+H2(g)��K2=
��ii��Fe(s)+H2O(g)FeO(s)+H2(g)��K2=![]() ������ʽi-ii��CO2(g)+H2(g)CO(g)+H2O(g)��K=
������ʽi-ii��CO2(g)+H2(g)CO(g)+H2O(g)��K=![]() =
=![]() ���¶�Ϊ973Kʱ��K=
���¶�Ϊ973Kʱ��K=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��

 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.01. | 0.008 | 0.007 | 0.007 | 0.007 |
��1����֪��K300����K350����д���÷�Ӧ��ƽ�ⳣ������ʽ��K=_________________�����ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
��2����ͼ�б�ʾNO2�ı仯��������____________________����O2��ʾ��0-2s�ڸ÷�Ӧ��ƽ������v=_______________��
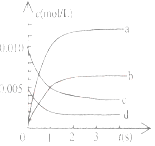
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����__________��
A��v(NO2)=2v(O2) B��������ѹǿ���ֲ���
C��v (NO)=2v��O2�� D���������ܶȱ��ֲ���
��4�����д�ʩ����ʹn(NO2)/n(NO)�������____��(����ĸ)
A�������¶� B���������
C�����ϳ���O2 D������He(g)��ʹ��ϵ��ѹǿ����
����Ŀ�����к͵ζ����ⶨij�ռ���Ʒ�Ĵ��ȣ��Ը���ʵ��ش��������⣺
��1��ȷ����8.2 g�������������������ʵ���Ʒ�����500 mL������Һ������ʱ����Ʒ�ɷ���_________(������ĸ)������
A��С�ձ��� B���ྻֽƬ�ϡ� C��������
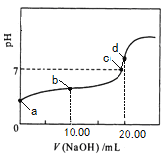
��2���ζ�ʱ����0.2000 mol/L�������Һ���ζ�������Һ����ѡ��_______(������ĸ)��ָʾ����
A������ B��ʯ�� C����̪
��3���ζ������У��۾�Ӧע��_______________________________�����÷�̪��ָʾ�����ζ��յ�ı�־��_____________________________________________��
��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����________mol/L���ռ���Ʒ�Ĵ�����_____________��
�ζ� ���� | ������Һ ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��5����δ�ô���Һ��ϴʢ�ű�����ĵζ��ܣ����ʹ�ⶨ���______����ƫ�ߡ���ƫ�͡�����Ӱ�족����