��Ŀ����
16�� ͭ���仯�����ڹ�ũҵ�������ճ����������Ź㷺����;����ش��������⣺
ͭ���仯�����ڹ�ũҵ�������ճ����������Ź㷺����;����ش��������⣺��1����̬ͭԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d104s1��
��2��NF3����NH3��F2��Cu���������·�Ӧֱ�ӵõ���4NH3+3F2$\frac{\underline{\;Cu\;}}{\;}$NF3+3NH4F
��������ѧ����ʽ�е�5�����������ľ���������abd������ţ���
a�����Ӿ��塡�� b�����Ӿ��塡�� c��ԭ�Ӿ��塡�� d����������
��F��N��O����Ԫ�صĵ�һ�������ɴ�С��˳��ΪF��N��O����Ԫ�ط��ű�ʾ��
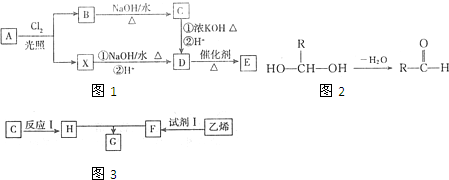
��3��ͭ����ͭԭ�ӵĶѻ���ʽΪ�����������ܶѻ���ÿ��ͭԭ����Χ���������ͭԭ����ĿΪ12��
��4��ijXԭ�ӵ���Χ�����Ų�ʽΪ3s23p5��ͭ��X�γɻ�����ľ�����ͼ��ʾ���ڵ����ͭԭ�ӣ���
�ٸþ���Ļ�ѧʽΪCuCl��
����֪�þ�����ܶ�Ϊ�� g•cm-3�������ӵ���������ֵΪNA����þ����о������ⳤΪ$\root{3}{\frac{4��99.5}{��NA}}$��1010pm��ֻд����ʽ��
��5���ϳɰ����յ�һ����Ҫ������ͭϴ����Ŀ������ͭҺ[���������ͭ��I������ˮ]���������������в�����CO��CO2�����壮ͭҺ����CO�ķ�Ӧ�Ƿ��ȷ�Ӧ���䷴Ӧ����ʽΪ��Cu��NH3��2Ac+CO+NH3?[Cu��NH3��3CO]Ac��Ac��ʾ�������
�����Ҫ���������Ӧ�ķ�Ӧ���ʣ����Բ�ȡ�Ĵ�ʩ��bc����ѡ���ţ�
a����ѹ������b������NH3��Ũ�ȡ�����c�����¡�����d����ʱ���߲���
��ͭҺ�����Ԫ���У�������Ԫ��ԭ�Ӱ뾶�Ӵ�С������˳��ΪC��N��O��H
��NH3�ĵ�ԭ�ӵ��ӻ�����Ϊsp3��
���� ��1��Cu��29��Ԫ�أ�ԭ�Ӻ��������Ϊ29�����ݺ�������Ų�������д��������Ų�ʽ��
��2���ٰ����������Լ�NF3Ϊ���Ӿ��壬ͭΪ�������壬NH4FΪ���Ӿ��壻
��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�
��3���ڽ�����������ܶѻ��У�����ÿ��ԭ����˵��������Χ��ԭ������֮ͬһ����������ԭ�Ӻ���һ�����������һ�����������ÿ��ԭ����Χ����12��ԭ����֮������
��4�����ݼ۵����Ų�ʽ�жϳ�Xԭ��ΪClԭ�ӣ�
�����þ�̯������ó���
�ڼ����һ�����������������û������Ħ���������ܶȼ���������߳���
��5��������Ũ�ȡ������¶ȵȣ�������Ӧ���ʣ�
��ͭҺ�����Ԫ���У�������Ԫ����H��C��N��O��Ԫ�أ�Hԭ�Ӱ뾶��С��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С��
�۸��ݼ۲���ӶԻ�������ȷ�����ӵ�ԭ���ӻ���ʽ���۲���ӶԸ���=�Ҽ�����+�µ��ӶԸ������ݴ˷������
��� �⣺��1��Cu��29��Ԫ�أ�ԭ�Ӻ��������Ϊ29����̬ԭ�Ӻ�������Ų�ʽΪ��1s22s22p63s23p63d104s1��
�ʴ�Ϊ��1s22s22p63s23p63d104s1��
��2�����ٰ����������Լ�NF3Ϊ���Ӿ��壬ͭΪ�������壬NH4FΪ���Ӿ��壬
�ʴ�Ϊ��abd��
��ͬһ����Ԫ���У�Ԫ�صĵ�һ����������ԭ����������������������ƣ�����IIA�塢��VA��Ԫ�صĵ�һ�����ܴ�������Ԫ�أ�F��N��O��Nԭ�������Ϊ������ṹ����Ϊ�ȶ����������ǵĵ�һ�����ܴ�С˳����I1��F����I1��N����I1��O�����ʴ�Ϊ��F��N��O��
��3���ڽ�����������ܶѻ��У�����ÿ��ԭ����˵��������Χ��ԭ������֮ͬһ����������ԭ�Ӻ���һ�����������һ�����������ÿ��ԭ����Χ����12��ԭ����֮����������ͭԭ��Ҳ����ˣ��ʴ�Ϊ��12��
��4���۵����Ų�ʽΪ3s23p5����Xԭ��ΪClԭ�ӣ�
���ɾ����ṹ��֪��Cuԭ�Ӵ��ھ����ڲ��������к���4��Cuԭ�ӣ�Clԭ�����ڶ����������ϣ������к���Clԭ����ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���ʻ�ѧʽΪCuCl��
�ʴ�Ϊ��CuCl��
��һ��������Ħ������Ϊ4��99.5g/mol������Ħ�����Ϊ$\frac{4��99.5g/mol}{��g/cm{\;}^{3}}$����һ���������Ϊ$\frac{4��99.5}{��•NA}$cm3���߳�Ϊ$\root{3}{\frac{4��99.5}{��NA}}$cm=$\root{3}{\frac{4��99.5}{��NA}}$��1010pm��
�ʴ�Ϊ��$\root{3}{\frac{4��99.5}{��NA}}$��1010��
��5��������Ũ�ȡ������¶ȵȣ�������Ӧ���ʣ���ѹ��Ӧ���ʼ�С����С������Ũ�ȣ���Ӧ���ʼ�С���ʴ�Ϊ��bc��
��ͭҺ�����Ԫ���У�������Ԫ����H��C��N��O��Ԫ�أ�Hԭ�Ӱ뾶��С��ͬ����Ԫ�ش�����ԭ�Ӱ뾶��С����ԭ�Ӱ뾶C��N��O��H���ʴ�Ϊ��C��N��O��H��
�۰����м۲���ӶԸ���=3+$\frac{1}{2}$��5-3��1��=4�Һ���1���µ��Ӷԣ�����Nԭ�Ӳ���sp3�ӻ����ʴ�Ϊ��sp3��
���� ���⿼���Ϊȫ�棬�漰�������Ų�ʽ���ӻ����͵��ж��Լ��йؾ���ļ���ȣ���������н�ǿ�ķ����Ժ����ԣ�ѧϰ��ע���ܽ������д�����Ų�ʽ������жϷ����ӻ���ʽ�Լ��йؾ������ȷ�����

 �Ͻ�ƽ���Ȿϵ�д�
�Ͻ�ƽ���Ȿϵ�д� ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�| A�� | ȩ�� | B�� | ���� | C�� | ϩ�� | D�� | ���� |
| A�� | Na2O2����������ߵĹ����� | |
| B�� | FeCl3��Һ����ͭ��ӡˢ��·������ | |
| C�� | ���ȼ�ˮ��������ϲ��������� | |
| D�� | 84����Һ���ڻ������� |
| A�� | �÷�Ӧ�漰���˹��ۼ������Ӽ��Ķ��Ѻ��γ� | |
| B�� | ��Ӧ�������е����������ڲ��������е������� | |
| C�� | �Ͽ�1molH-H����1mol��Cl-Cl���������յ���������С���γ�1molH-Cl�������ų������� | |
| D�� | �÷�Ӧ�У�����ת��Ϊ��ѧ�� |
| A�� | ̼��ˮ��Ӧ����һ����̼������������131.5kJ���� | |
| B�� | 1mol̼��1molˮ��Ӧ����һ����̼������������131.5kJ���� | |
| C�� | 1mol��̬̼��1molˮ������Ӧ����һ����̼���������������131.5kJ���� | |
| D�� | 1����̬̼ԭ�Ӻ�1����ˮ������Ӧ����131.5kJ���� |
| A�� | ��H2SO4 | B�� | ��NH4Cl | C�� | ���� | D�� | ��ѹ |
| A�� | �����½�64gͭƬͶ�����Ũ������ | |
| B�� | ��5.5gMnO2��ĩ�м���20mL2mol•L-1˫��ˮ | |
| C�� | ��10mL3mol•L-1�������м���5.6g�� | |
| D�� | ����Ba��OH��2��NaOH��0.1mol�Ļ����Һ��ͨ������11.2CO2 |
| A�� | �÷�Ӧ�ﵽƽ��ı�־��X��Ũ�Ȳ��ٷ��仯 | |
| B�� | �����¶���ʹ�÷�Ӧ�����ʼӿ� | |
| C�� | ������Z��ʾ�ķ�Ӧ����Ϊ1.2mol/��L•s�� | |
| D�� | 2sʱY��Ũ��Ϊ0.7mol/L |
 ��C�й����ŵ�������ȩ����
��C�й����ŵ�������ȩ���� ��
��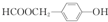 ��
��