��Ŀ����
����Ŀ������(COCl2)��ũҩ��ҽҩ���������ϵȷ��涼�й㷺Ӧ�ã�����������Ϊ��ɫ�����и���ζ������ʱΪ����ɫҺ�壬��ѧ���ʲ��ȶ�����ˮѸ��ˮ�⣬�����Ȼ��⡣ijʵ��С����������ʵ��װ�úϳɹ��������ù�����Ũ��ˮ��Ӧ�Ʊ�����[CO(NH2)2]����Ҫʵ��װ��(�г�װ����ȥ)�������������£�
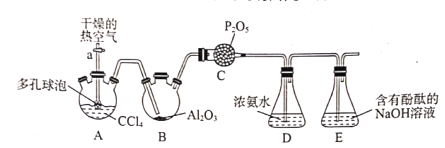
�ٰ���ͼ����װ�ã�����װ�õ������ԣ�Ȼ���װʵ��ҩƷ��
�ڴ���a.����A�л���ͨ�������ȿ�����
��һ��ʱ���װ��D����Һ����ֲַ������һ��Һ�Ϸ��д�����ɫ������
��֪��3CC14+Al2O3=3COCl2+2AlC13
�ش��������⣺
(1)��������װ�õ������Եķ�����___��
(2)�������ͨ�������ȿ���������Ϊ___��
(3)װ��C������___��
(4)װ��D�з�Ӧ����������[CO(NH2)2���⣬����NH4Cl���ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ___����
(5)����װ��D�л��Һ�IJ�������Ϊ___��
(6)װ��E��������NaOH��Һ�������Ĺ�����Ӧ�����ӷ���ʽΪ��___��
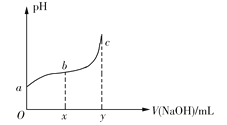
(7)ʵ���D����Һ�������ᾧ�������ؽᾧ�ķ����õ����ؾ���(����������NH4Cl����)���ⶨ���þ��������صİٷֺ����ķ�������7.07g��������������ȫת��Ϊ�������ð�����100mL2.00molL-1��������Һ��ȫ���գ�ȡ����Һ10mL��0.4000molL-1���������Ʊ���Һ�ζ���
�ٵ���ζ��յ�ʱ��������������45.00mL����þ��������ص���������Ϊ___(����3λ��Ч����)��
�����ζ�ǰδ���������Ʊ���Һ��ϴ�ζ��ܣ����ø��徧�����ص���������___(ѡ����ƫ��������ƫС��)��
���𰸡��رջ���a����װ��ĩ�˵��ܲ���ʢˮ���ձ��У�����װ��A���۲�ĩ�˵��ܿ������ݲ�����ֹͣ���ȣ�ĩ�˵��ܿ���һ��ˮ�� ��������CCl4�����ҽ��ȵ�CC14�������������Bװ�÷�����Ӧ ��ֹDװ���е�ˮ����װ��B�����¹�������ˮ�ⷴӦ COC12+4NH3H2O=CO(NH2)2+2NH4Cl+4H2O ��Һ COCl2+4OH-=CO32-+2Cl-+2H2O 84.9% ƫС
��������
��ʵ��Ŀ���Ǻϳɹ��������ù�����Ũ��ˮ��Ӧ�Ʊ�����[CO(NH2)2]��������ѧ���ʲ��ȶ�����ˮѸ��ˮ�⣬�����Ʊ�����ʱҪ������ϵ����ø�����ȿ����������������Ȼ�̼�������䴵��Bװ������Al2O3���з�Ӧ�Ʊ�������Cװ�ÿ��Է�ֹˮ��������Bװ�ã�����ͨ��Ũ��ˮ�з�Ӧ�Ʊ�[CO(NH2)2]��D���б������������Ȼ�̼���������Ȼ�̼������ˮ�����Գ��ֲַ�����δ��Ӧ�Ĺ����ڻ��Һ�Ϸ���ˮ������Ӧ����HCl��CO2��HCl�����ӷ����İ�����Ӧ�����Ȼ�泥����Բ����������̣�װ��E��������β����
(7)�ζ�ԭ��Ϊ�Ƚ���Ʒ�е�NԪ��ȫ��ת��Ϊ�������������գ�Ȼ����NaOH��Һ�ζ�ʣ�������������Ӷ�ȷ��NԪ�ص������ٸ����غ㷨����ȷ����Ʒ�����صĺ�����
(1)����ͨ�����ȷ�����װ�õ������ԣ��������Ϊ���رջ���a����װ��ĩ�˵��ܲ���ʢˮ���ձ��У�����װ��A���۲�ĩ�˵��ܿ������ݲ�����ֹͣ���ȣ�ĩ�˵��ܿ���һ��ˮ����˵�����������ã�
(2)���ݷ�����֪ͨ������ȿ���������Ϊ��������CCl4�����ҽ��ȵ�CC14�������������Bװ�÷�����Ӧ��
(3)������ѧ���ʲ��ȶ�����ˮѸ��ˮ�⣬װ��C��������Ҫ�Ƿ�ֹDװ���е�ˮ��������װ��B�����¹�������ˮ�ⷴӦ��
(4)D�з�Ӧ��ΪCOCl2��һˮ�ϰ���������[CO(NH2)2]��NH4Cl�ȣ�����Ԫ���غ�ɵ÷���ʽΪCOC12+4NH3H2O=CO(NH2)2+2NH4Cl+4H2O��
(5)װ��D����Һ�ֲ㣬��ͨ����Һ�ķ������룻
(6)������ˮˮ�����HCl��CO2��HCl��NaOH��Ӧ����NaCl��ˮ��CO2��NaOH��Ӧ����Na2CO3��ˮ�����Թ�����NaOH��Һ��Ӧ�����ӷ���ʽΪCOCl2+4OH-=CO32-+2Cl-+2H2O��
(7)�ٵ���ζ��յ�ʱ��������������45.00mL����10mL����Һʣ��n(H+)=0.045L��0.4000mol/L=0.018mol�������õİ���n(NH3)=0.1L��2.00mol/L��2-![]() =0.22mol���辧����n([CO(NH2)2])=x mol��n(NH4+)=y mol��
=0.22mol���辧����n([CO(NH2)2])=x mol��n(NH4+)=y mol��
����Ԫ���غ�ɵ�![]() �����x=0.1mol��y=0.02mol���������ص���������Ϊ
�����x=0.1mol��y=0.02mol���������ص���������Ϊ![]() ��100%=84.9%��
��100%=84.9%��
�����ζ�ǰδ���������Ʊ���Һ��ϴ�ζ���,��ʹ���ĵ��������Ʊ���Һƫ�࣬���øþ��������ص���������ƫС��

 ÿ��10���ӿ�����������������ϵ�д�
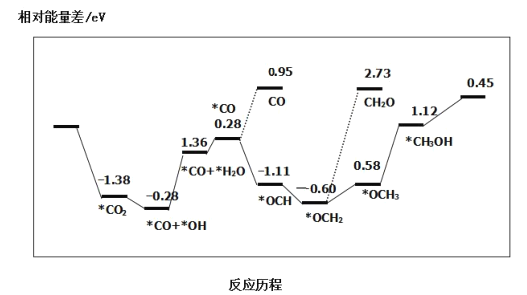
ÿ��10���ӿ�����������������ϵ�д�����Ŀ����CO2Ӧ�����������ȼ�ϼ״������ܻ�������ЧӦ��Ӱ�죬����Ϊ��Դ���Ʊ������µ���������ϳɷ�ӦΪCO2(g)+3H2(g)![]() CH3OH(g)+H2O(g)���ش��������⣺
CH3OH(g)+H2O(g)���ش��������⣺
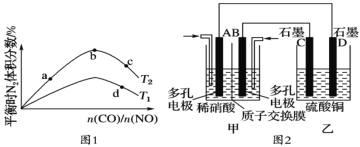
��1����ͼΪCO2ƽ��ת���ʺ��¶ȡ�ѹǿ�Ĺ�ϵ������ѹǿ�ֱ�Ϊ3.0MPa��4.0MPa��5.0MPa����ͼ��֪���÷�ӦΪ_______________��Ӧ������ȡ�������")����CO2�ij�ʼŨ��ΪcomolL-1������5.0MPaʱ�����ݼ���÷�Ӧ��ƽ�ⳣ��K(240k)=_______________ (�г�����ʽ���ɣ�������4.0MPaʱ��СͶ�ϱȣ���CO2��ƽ��ת�������߿���λ��II�ߵ�_______________����Ϸ������·���)��

��2�����ö�����̼�Ƶõļ״���������ȡ�װ����䷴Ӧԭ��ΪCH3OH(g)+NH3(g)![]() CH3NH2(g)+H2O(g)��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
CH3NH2(g)+H2O(g)��H����֪�÷�Ӧ����ػ�ѧ���ļ����������£�
���ۼ� | C��O | H��O | N��H | C��N |
����/k.Jmol-1 | 351 | 463 | 393 | 293 |
��÷�Ӧ�ġ�H=_______________k.Jmol-1 ��
��3����֪����CO(g)+NO2(g)![]() CO2(g)+NO(g) ��H1=-226kJrnol-1
CO2(g)+NO(g) ��H1=-226kJrnol-1
��N2(g)+2O2(g)![]() 2NO2(g)��H2=+68kJmol-1
2NO2(g)��H2=+68kJmol-1
��N2(g)+O2(g)![]() 2NO(g) ��H3=+183kJmol-1
2NO(g) ��H3=+183kJmol-1
��2CO(g)+2NO(g)![]() 2CO2(g)+N2(g) ��H=_______________kJmol-1��
2CO2(g)+N2(g) ��H=_______________kJmol-1��
��4��һ���¶��£����д�ʩһ���ܼӿ췴ӦCO2(g)+3H2��g)![]() CH3OH(g)+H2O(g)�����ʵ���_______________����ѡ����ĸ����
CH3OH(g)+H2O(g)�����ʵ���_______________����ѡ����ĸ����
A.��ʱ��ȥ�״� B.�Ľ����� C.��߷�Ӧ��Ũ�� D.��������ѹǿ
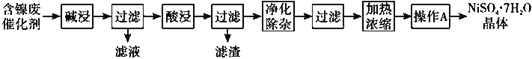
��5������������ѡ������NiO- Al2O3��Ϊ��������ҵ�ϳ���Ni(NO3)2
����Ŀ��ij����(Ni)�ϴ�������Ҫ����Ni��������Al��Al2O3��Fe�������������ᡢ������ʡ����ֽ�����������Ksp����ֵ���±���ʾ��
��ѧʽ | Fe(OH)2 | Fe(OH)3 | Al(OH)3 | Ni(OH)2 |
Ksp����ֵ | 10-17 | 10-39 | 10-34 | 10-15 |
���ú����ϴ����Ʊ�NiSO4��7H2O���壬������ͼ���£�
�ش��������⣺
��1���������ʱ������Ӧ�����ӷ���ʽΪ2A1+2OH-+2H2O=2AlO2��+3H2����_________��
��2�����������ʹ�õ���Ϊ_____________��
��3�������������������H2O2��Һ����������__________________________��Ȼ�����pHʹ��Һ����Ԫ��ǡ����ȫ��������ʱ��pHΪ____________������1λС������
��4��������A��Ϊ_____�����ˡ�ϴ�ӡ�������ò�Ʒ��
��5��NiSO4��NaOH��Һ�пɱ�NaClO����ΪNiOOH���÷�Ӧ�Ļ�ѧ����ʽΪ_______��
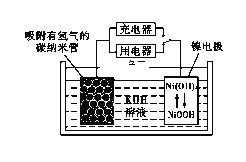
��6��NiOOH����Ϊ�����صĵ缫���ϣ��õ�صĹ���ԭ������ͼ��ʾ������ʱ�������ĵ缫��ӦʽΪ____________��