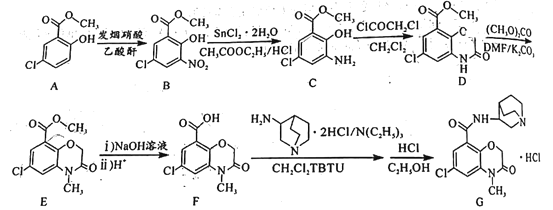
��Ŀ����
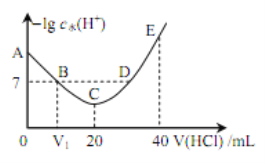
����Ŀ�������£���20 mL x mol��L��1 CH3COOH��Һ����μ�������ʵ���Ũ�ȵ�NaOH��Һ�����Һ��pH��NaOH��Һ�������V���ı仯��ϵ��ͼ��ʾ�������¶ȱ仯��������˵���в���ȷ���ǣ� ��
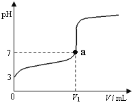
A. ���� CH3COOH��Һ�У�c(H+)��1��10��3 mol��L��1
B. ͼ��V1 <20 mL
C. a���Ӧ����Һ�У�c (CH3COO��)��c (Na+��
D. ������NaOH��Һ�����Ϊ20 mLʱ����Һ�У�c (CH3COOH) + c (H+)��c (OH����
���𰸡�D
��������
A����ͼ���֪������NaOH��Һ֮ǰ��������Һ��pH=3��˵����Һ��c(H+)=1��10-3molL-1����A��ȷ��
B�������ʵ���Ũ�ȶ�ΪxmolL-1��CH3COOH��NaOH��Һ�������ϣ����Ϻ�ǡ������CH3COONa��CH3COONaΪǿ�������Σ�ˮ��ʼ��ԣ�����Һ�����ԣ������NaOH��Һ�����V1��20 mL����B��ȷ��
C����Һ�д��ڵ���غ㣺c(CH3COO-)+c(OH-)=c(H+)+c(Na+)��a��ʱ��Һ��pH=7����c(OH-)=c(H+)������c(CH3COO-)=c(Na+)����C��ȷ��
D��������NaOH��Һ�����Ϊ20mLʱ��ǡ�÷�Ӧ����CH3COONa����Һ�д��������غ㣺c(CH3COO-)+c(CH3COOH)=c(Na+)�����ڵ���غ㣺c(CH3COO-)+c(OH-)=c(H+)+c(Na+)����֪��ʽ�ɵã�c(OH-)=c(H+)+c(CH3COOH)����D����
�ʴ�ΪD��