��Ŀ����
A��B��C��D��E��F���ڶ���������Ԫ�أ�����AԪ��ԭ���Ƕ�������ԭ�Ӱ뾶����ԭ�ӣ�B����С��ԭ�ӣ�CԪ��ԭ�ӵ�����������Ϊm������������Ϊn��DԪ��ԭ�ӵ�L�������Ϊm+n��M�������Ϊm-n��EԪ��ԭ�ӵ�������������������Ӳ������������ڱ�����DԪ�����ڣ� FԪ��ԭ�ӵĺ����������CԪ��ԭ�ӵ�2����A��B��C����Ԫ�ؿ���ɻ�����X��C��D����ɻ�����Y��C��E����ɻ�����Z��
��1��д��EԪ�ط��� ��A��ԭ�ӽṹʾ��ͼ ��
��2��F��Ԫ�����ڱ���λ��Ϊ ������se��������������Ԫ�أ���Fͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪ ��
��3��A��F����Ԫ�����γɵļ������У���Mg2+������ͬ���Ӳ�ṹ���� �������ӷ��ű�ʾ���������Ӱ뾶�ɴ�С��˳��Ϊ ��
��4��������Z��X��ˮ��Һ��Ӧ�����ӷ���ʽΪ ��
��5��������Y��;�㷺�����о�����һ����; ��
��1��д��EԪ�ط���
��2��F��Ԫ�����ڱ���λ��Ϊ
��3��A��F����Ԫ�����γɵļ������У���Mg2+������ͬ���Ӳ�ṹ����
��4��������Z��X��ˮ��Һ��Ӧ�����ӷ���ʽΪ
��5��������Y��;�㷺�����о�����һ����;
������A��B��C��D��E��F���ڶ���������Ԫ�أ�����AԪ��ԭ���Ƕ�������ԭ�Ӱ뾶����ԭ�ӣ���AΪNaԪ�أ�B����С��ԭ�ӣ���BΪHԪ�أ�CԪ��ԭ�ӵ�����������Ϊm������������Ϊn��DԪ��ԭ�ӵ�L�������Ϊm+n��M�������Ϊm-n����m+n=8����CԪ��ԭ�ӵĴ����Ϊ��1���Ӳ㣬��n=2����m=6����CΪ��Ԫ�أ�D����������Ϊ6-2=4����DΪSiԪ�أ�EԪ��ԭ�ӵ�������������������Ӳ�����������������Ϊ3����EΪAlԪ�أ�FԪ��ԭ�ӵĺ����������CԪ��ԭ�ӵ�2������Fԭ�Ӻ��������Ϊ16����FΪ��Ԫ�أ�A��B��C����Ԫ�ؿ���ɻ�����XΪNaOH��C��D����ɻ�����YΪSiO2��C��E����ɻ�����ZΪAl2O3���ݴ˽��
����⣺A��B��C��D��E��F���ڶ���������Ԫ�أ�����AԪ��ԭ���Ƕ�������ԭ�Ӱ뾶����ԭ�ӣ���AΪNaԪ�أ�B����С��ԭ�ӣ���BΪHԪ�أ�CԪ��ԭ�ӵ�����������Ϊm������������Ϊn��DԪ��ԭ�ӵ�L�������Ϊm+n��M�������Ϊm-n����m+n=8����CԪ��ԭ�ӵĴ����Ϊ��1���Ӳ㣬��n=2����m=6����CΪ��Ԫ�أ�D����������Ϊ6-2=4����DΪSiԪ�أ�EԪ��ԭ�ӵ�������������������Ӳ�����������������Ϊ3����EΪAlԪ�أ�FԪ��ԭ�ӵĺ����������CԪ��ԭ�ӵ�2������Fԭ�Ӻ��������Ϊ16����FΪ��Ԫ�أ�A��B��C����Ԫ�ؿ���ɻ�����XΪNaOH��C��D����ɻ�����YΪSiO2��C��E����ɻ�����ZΪAl2O3��
��1��������������֪��EԪ�ط���ΪAl��AΪNaԪ�أ���ԭ�ӽṹʾ��ͼΪ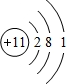 ��
��
�ʴ�Ϊ��Al��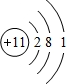 ��
��
��2��FΪ��Ԫ�أ���Ԫ�����ڱ���λ��Ϊ�������ڢ�A�壻����se��������������Ԫ�أ���Fͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪH2SeO4��
�ʴ�Ϊ���������ڢ�A�壻H2SeO4��
��3��A��F����Ԫ�����γɵļ������У���Mg2+������ͬ���Ӳ�ṹ����Na+��Al3-��O2-�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶�ɴ�С��˳��ΪO2-��Na+��Al3-��
�ʴ�Ϊ��Na+��Al3-��O2-��O2-��Na+��Al3-��
��4�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��5��YΪSiO2������;�㷺����������������ά����ѧ�����ȣ�
�ʴ�Ϊ��������ά����ѧ�����ȣ�
��1��������������֪��EԪ�ط���ΪAl��AΪNaԪ�أ���ԭ�ӽṹʾ��ͼΪ
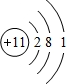 ��
���ʴ�Ϊ��Al��
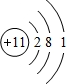 ��
����2��FΪ��Ԫ�أ���Ԫ�����ڱ���λ��Ϊ�������ڢ�A�壻����se��������������Ԫ�أ���Fͬһ���壬������������Ӧ��ˮ���ﻯѧʽΪH2SeO4��
�ʴ�Ϊ���������ڢ�A�壻H2SeO4��
��3��A��F����Ԫ�����γɵļ������У���Mg2+������ͬ���Ӳ�ṹ����Na+��Al3-��O2-�����Ӳ�ṹ��ͬ�˵����Խ�����Ӱ뾶ԽС�������Ӱ뾶�ɴ�С��˳��ΪO2-��Na+��Al3-��
�ʴ�Ϊ��Na+��Al3-��O2-��O2-��Na+��Al3-��
��4�����������������Ʒ�Ӧ����ƫ��������ˮ����Ӧ���ӷ���ʽΪAl2O3+2OH-=2AlO2-+H2O��
�ʴ�Ϊ��Al2O3+2OH-=2AlO2-+H2O��
��5��YΪSiO2������;�㷺����������������ά����ѧ�����ȣ�
�ʴ�Ϊ��������ά����ѧ�����ȣ�
���������⿼��ṹ����λ�ù�ϵ���漰��ѧ���Ԫ�ػ�������������;�ȣ��ѶȲ����ƶ�Ԫ���ǽ���Ĺؼ���ע��Ի���֪ʶ���������գ�

��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�