��Ŀ����
A��B��C��D��E��FΪ���ֶ�����Ԫ�أ����Ǻ˵�������ε�������֪��Bԭ�Ӻ�������ԳɶԵ��ӣ��ҵ���������Eԭ��������1/2��F��ͬ����Ԫ����ԭ�Ӱ뾶��С��Ԫ�أ�D2-��E2+�ĵ��Ӳ�ṹ��ͬ��B��D�����γ���ԭ�ӻ�����ס�A�Ƿǽ���Ԫ�أ���A��C��F���γ����ӻ������ҡ���ش�
��1��C���ʵĵ���ʽΪ__________��FԪ��ԭ�ӵĺ�������Ų�ʽΪ__________��
��2��B��C��D��Ԫ�ص���̬�⻯����ȶ�����ǿ������˳����____________________��
��3�����������к��еĻ�ѧ����____________________��
��4���������Ϊ����ʱ�ľ�����������__________________��E������һ�����������Ӧ�Ļ�ѧ����ʽΪ____________________��
��1��C���ʵĵ���ʽΪ__________��FԪ��ԭ�ӵĺ�������Ų�ʽΪ__________��
��2��B��C��D��Ԫ�ص���̬�⻯����ȶ�����ǿ������˳����____________________��
��3�����������к��еĻ�ѧ����____________________��
��4���������Ϊ����ʱ�ľ�����������__________________��E������һ�����������Ӧ�Ļ�ѧ����ʽΪ____________________��
��1��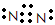 ��1s22s22p63s23p5
��1s22s22p63s23p5
��2��H2O��NH3��CH4
��3�����Ӽ������ۼ�����λ��
��4�����Ӿ��壻2Mg+CO2 2MgO+C
2MgO+C
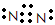 ��1s22s22p63s23p5
��1s22s22p63s23p5 ��2��H2O��NH3��CH4
��3�����Ӽ������ۼ�����λ��
��4�����Ӿ��壻2Mg+CO2
 2MgO+C
2MgO+C 
��ϰ��ϵ�д�
�����Ŀ
 [��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�
[��ѧ/ѡ��/���ʽṹ������]A��B��C��D��E���ֶ�����Ԫ�أ�ԭ��������������Ԫ�ض�Ӧ�ĵ��ʾ�Ϊ���壮A��C��E��Ԫ�ص�ԭ�Ӻ����ֻ��2��δ�ɶԵ��ӣ�B��EԪ�ص�ԭ������֮�͵���C��DԪ�ص�ԭ������֮�ͣ�