��Ŀ����
����Ŀ���Ժ��ܷϴ���(��Ҫ�ɷ�ΪCo��Fe��SiO2)Ϊԭ����ȡ���������ܵ�����������
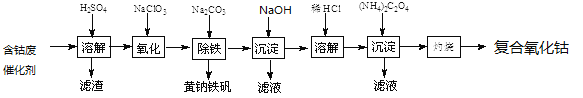
��1����H2SO4�ܽ��������õ���������_________(�ѧʽ)��������ϴ��2��3�����ٽ�ϴҺ����Һ�ϲ���Ŀ����____________________��
��2���ڼ��Ƚ��������¼���NaClO3����Fe2+������Fe3+����Ӧ�����ӷ���ʽ��___________________��
��3����֪�����軯�صĻ�ѧʽΪK3[Fe(CN)6]�������軯�صĻ�ѧʽΪK4[Fe(CN)6]��
3Fe2++2[Fe(CN)6]3-=Fe3[Fe(CN)6]2��(��ɫ����)
4Fe3++3[Fe(CN)6]4-=Fe4[Fe(CN)6]3��(��ɫ����)
ȷ��Fe2+�Ƿ�������ȫ�ķ�����__________________��(����ѡ����Լ������軯����Һ�������軯����Һ���ۡ�KSCN��Һ)
��4�������������Һ�м���������Na2CO3������ȣ�ʹ֮���ɻ�������[Na2Fe6(SO4)4(OH)12]������д���÷�Ӧ�����ӷ���ʽ��_______________________��
��5�����������ĵ���ƽ�ⳣ���ĸ���������pK��ʾ�������±��������ж�(NH4)2C2O4��Һ�и����ӵ�Ũ���ɴ�С��˳��Ϊ__________________��
H2C2O4 | pKa1= l.25��pKa2=4.13 |
NH3��H2O | pKb=4.76 |
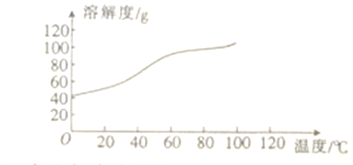
��6����֪CoCl2���ܽ��������ͼ��ʾ�����ʽ̼�����м�������ϡ������ȱ߽�������ȫ�ܽ������ȹ�����ԭ����_____________________��
��7��ȷ��ȡ1.470gCoC2O4���ڿ����г�����յ�0.814g���������ܣ�д�����������ܵĻ�ѧʽ��_________________________��
���𰸡� SiO2 �����Ԫ�ص������� 6Fe2++6H++ClO3-![]() 6Fe3++Cl-+3H2O ȡ�������������Һ���Թ��еμӼ������軯����Һ��������ɫ�������ɣ���˵��Fe2+��ȫ�������� 6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2�� c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-) ��ֹ���¶Ƚ�����CoCl2�������� Co5O7
6Fe3++Cl-+3H2O ȡ�������������Һ���Թ��еμӼ������軯����Һ��������ɫ�������ɣ���˵��Fe2+��ȫ�������� 6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2�� c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-) ��ֹ���¶Ƚ�����CoCl2�������� Co5O7
����������1���ܷϴ�������ϡ���ᣬ����Co+H2SO4=CoSO4+H2����Fe+H2SO4=FeSO4+H2�������������Dz��ܵĶ���������ϴҺ����Һ�ϲ������ϴ�Ӻ���Һ���ܵ������ʣ��ʴ�Ϊ��SiO2������ܵ�Ԫ�ص������ʣ�
��2���������ӱ���������������������ӣ�1mol����������ʧȥ1mol�ĵ��ӣ���1mol����������ӵõ�6mol�ĵ��ӣ����ݵ��ӵ�ʧ�غ㣬��֪���ӷ���ʽΪ��6Fe2++6H++ClO3-![]() 6Fe3++Cl-+3H2O��
6Fe3++Cl-+3H2O��
��3��ȡ���������Һ�������Թ��У��μӼ������軯����Һ��������ɫ�������ɣ���Fe2+��ȫ����������
��4��������������̼���Ʒ���˫ˮ��õ��������������ӷ���ʽΪ��6Fe3++4SO42-+6H2O+2Na++6CO32-=Na2Fe6(SO4)4(OH)12��+6CO2����
��5������pK�Ĵ�С��ϵ�������жϳ����볣���Ĵ�С��ϵΪKa1> Ka2>Kb�����Զ�Ӧ���ӵ�ˮ��̶ȴ�СΪNH4+>C2O42->HC2O4-��ˮ��̶�Խ����Һ��ʣ������Ũ�Ⱦ�ԽС��������Һ�е�����Ũ�ȴ�С˳��Ϊ��c(NH4+)>c(C2O42-)>c(H+)>c(HC2O4-)>c(OH-)
��6��CoCl2���ܽ�����߿�֪�����¶ȵ����ߣ�CoCl2���ܽ���������Գ��ȹ��ˣ���ֹ�¶Ƚ����Ȼ����������ʴ�Ϊ����ֹ���¶Ƚ��ͣ�CoCl2����������
��7��CoC2O4������Ϊ1.470g�������ʵ���Ϊ0.01mol��CoԪ������Ϊ0.59g��������������Ϊ0.814g������������Ԫ������Ϊ0.814g-0.59g=0.224g������������Coԭ����Oԭ�����ʵ���֮��Ϊ0.01mol��![]() ��5��7����Co������ΪCo5O7���ʴ�Ϊ��Co5O7��
��5��7����Co������ΪCo5O7���ʴ�Ϊ��Co5O7��

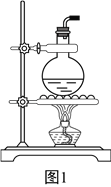
����Ŀ�����������(H2NCOONH4)��һ���ֽ⡢��ˮ��İ�ɫ������ij�о�С�����������ƹ��塢Ũ��ˮ���ɱ���Ϊԭ���Ʊ���������淋�ʵ��װ����ͼ��ʾ������Ҫ��Ӧ��ԭ��Ϊ2NH3(g)+CO2(g)![]() NH2COONH4(s) ��H<O��
NH2COONH4(s) ��H<O��
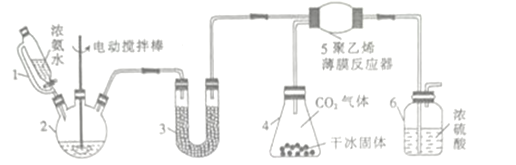
��1������2��������________������3��ʢװ�Ĺ�����_________����������______________��
��2������6��һ�������ǿ���ԭ��������Ӧ����ϵ����ַ�Ӧ������Ӧ���ڹ۲쵽װ����Ũ�����в������ݣ���Ӧ��______________(�����ӿ����� �������������ı���)�������������ʡ�
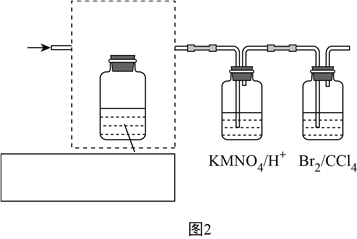
��3����һ���Ʊ��������ᰱ�ķ�Ӧװ��(Һ��ʯ����CCl4���䵱���Խ���)��ͼ��ʾ��
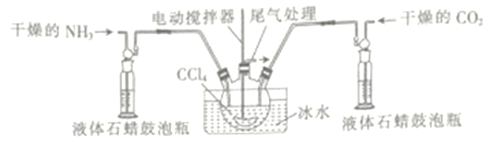
��Һ��ʯ������ƿ��������_____________________ ��
�����ޱ�ˮ�����������ֽ���������[CO(NH)2]2��д��������������ȷֽ�Ļ�ѧ����ʽ��_______________________��
�۵�CCl4Һ���в����϶ྦྷ��������ʱ������ֹͣ��Ӧ�����˷���õ��ֲ�Ʒ��Ϊ�˽����ôֲ�Ʒ�������ɲ�ȡ�ķ�����_______________(����ĸ)��
A.���� B.����Ⱥ�� C.��ѹ���Ⱥ��
��4���Ƶõİ���������п��ܺ���̼�������̼����е�һ�ֻ���������(�����ǰ����������ˮ�ķ�Ӧ)��
����Ʒ������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡ������BaCl2��Һ������ʯ��ˮ��AgNO3��Һ��ϡ���ᡣ
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ����������Ʒ���Թ�������������ˮ�������ܽ� | �õ���ɫ��Һ |
����2�����Թ��м��������BaCl2��Һ������ | ����Һ������ǣ���֤�������в���̼��� |
����3�����Թ��м�������___________ | _____________________________�� ��֤�������к���̼����� |
�ڸ��ݢٵĽ�����ȡ15.8g�������ᰱ��Ʒ������������������Һ��ִ�����������ϴ�ӡ������ó�������Ϊ1.97g������Ʒ�а�������淋���������Ϊ_________________��