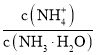��Ŀ����
����Ŀ����Ҫ��ش��������⣺
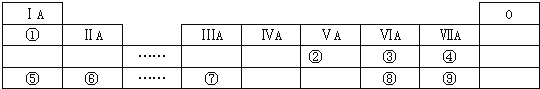
(1)ʵ�����г���NaOH��Һ������ϴ�����ᴿ����100mL![]() ��NaOH��Һ���ձ�״����
��NaOH��Һ���ձ�״����![]()
![]() ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ______
ʱ��������Һ�и�����Ũ���ɴ�С��˳��Ϊ______
(2)��������һ�������![]() ������Һ�м�ˮϡ�ͺ�����˵����ȷ����______��
������Һ�м�ˮϡ�ͺ�����˵����ȷ����______��
A.��Һ�е������ӵ���Ŀ���� ![]() ����ĵ���̶�����
����ĵ���̶�����![]() ������
������
C.��Һ�� ����
���� ![]() ��Һ��
��Һ�� ��С
��С
(3)�ٳ����½�![]() ϡ����
ϡ����![]() ��
��![]() NaOH��Һ
NaOH��Һ![]() mL���ϣ�������Һ��pHΪ1����
mL���ϣ�������Һ��pHΪ1����![]() ��
��![]() ______
______![]() ��Һ����仯���Բ�ѯ��
��Һ����仯���Բ�ѯ��
�ڳ���������Һ��![]() ��HA��Һ
��HA��Һ![]() ��
��![]() ��NaOH��Һ
��NaOH��Һ![]() ��϶��ã�������˵����ȷ����______
��϶��ã�������˵����ȷ����______
A.����Ӧ����Һ�����ԣ���![]()
B.��![]() ����Ӧ����ҺpHһ������7
����Ӧ����ҺpHһ������7
C.����Ӧ����Һ�����ԣ���![]() һ������
һ������![]()
D.����Ӧ����Һ�ʼ��ԣ���![]() һ��С��
һ��С��![]()
(4)�����£�Ũ�Ⱦ�Ϊ![]() ������������Һ��pHֵ���±���ʾ��
������������Һ��pHֵ���±���ʾ��
���� |
|
|
| NaClO | NaCN |
pH |
|
|
|
|
|
�ٸ��ݱ������ݣ���Ũ�Ⱦ�Ϊ![]() ���������������Һ�ֱ�ϡ��100����pH�仯��С����______
���������������Һ�ֱ�ϡ��100����pH�仯��С����______
A.HCN![]()
![]()
![]()
�ڸ����������ݣ��ж����з�Ӧ���Գ�������______��
A.![]()
![]()
C.![]()
![]()
(5)�������ӿ�ʼ����ʱ��pH���±���
���� |
|
|
|
pH |
|
|
|
������ͬŨ��![]() ��
��![]() ��
��![]() ���ӵ���Һ�еμ�NaOH��Һʱ��______
���ӵ���Һ�еμ�NaOH��Һʱ��______![]() �����ӷ���
�����ӷ���![]() �ȳ�����
�ȳ�����![]() ______
______![]() ����
����![]() ������
������![]() ������
������![]() ��
��![]() ��
��
���𰸡�![]() CD 1��1 AD A AB
CD 1��1 AD A AB ![]()
![]()
��������
![]() ��
��![]() �������ɵ�̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy����
�������ɵ�̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy����![]() �����
�����![]() ����̼���ƺ�̼�����Ƶ����ʵ�������
����̼���ƺ�̼�����Ƶ����ʵ�������![]() ��̼���ƺ�̼�����ƻ����Һ�ʼ��ԣ�̼�������ˮ��̶ȴ���̼��������ӣ��ٽ�������غ��жϣ�
��̼���ƺ�̼�����ƻ����Һ�ʼ��ԣ�̼�������ˮ��̶ȴ���̼��������ӣ��ٽ�������غ��жϣ�
![]() ��ˮϡ�ʹٽ�������룬��Һ�д��������Ũ�ȡ��������Ũ�ȡ�������Ũ�ȶ���С������������Ũ������
��ˮϡ�ʹٽ�������룬��Һ�д��������Ũ�ȡ��������Ũ�ȡ�������Ũ�ȶ���С������������Ũ������
![]() ������Һ��pHֵΪ1����Һ��������Ũ��Ϊ
������Һ��pHֵΪ1����Һ��������Ũ��Ϊ![]() ��˵�������ӹ�������������ϡ���������������Һ�����ʽ�����
��˵�������ӹ�������������ϡ���������������Һ�����ʽ�����![]() ��
��![]() �ı�ֵ��
�ı�ֵ��
![]() ������Ӧ����Һ�����ԣ���
������Ӧ����Һ�����ԣ���![]() ��
��
B����![]() ����Ӧ����ҺpH����7��С��7��
����Ӧ����ҺpH����7��С��7��
C������Ӧ����Һ�����ԣ�![]() ���ܵ���
���ܵ���![]() ��
��
D�������Һ�ʼ��ԣ�����Һ�����Ǽ������Һ��Ҳ����ֻ������Һ��
![]() ��ͬŨ�ȵ�������Һ���������Խ�������������ˮ��̶�Խ����������ҺpHȷ�����ǿ������ͬŨ�ȵIJ�ͬ�ᣬ��ˮϡ�ʹٽ�������룬��ϡ����ͬ�ı������������Խ��������Һϡ������pH�仯ԽС��
��ͬŨ�ȵ�������Һ���������Խ�������������ˮ��̶�Խ����������ҺpHȷ�����ǿ������ͬŨ�ȵIJ�ͬ�ᣬ��ˮϡ�ʹٽ�������룬��ϡ����ͬ�ı������������Խ��������Һϡ������pH�仯ԽС��
![]() �������ˮ��̶�Խǿ�����������Խ�������ǿ����ȡ���������
�������ˮ��̶�Խǿ�����������Խ�������ǿ����ȡ���������
![]() ���ɳ�����Ҫ��pHԽС����������ȳ������������ӻ������������ܶȻ�������
���ɳ�����Ҫ��pHԽС����������ȳ������������ӻ������������ܶȻ�������
![]() ��
��![]() �������ɵ�̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy����
�������ɵ�̼���Ƶ����ʵ���Ϊx��̼�����Ƶ����ʵ���Ϊy���� �����
�����![]() ����̼���ƺ�̼�����Ƶ����ʵ�������
����̼���ƺ�̼�����Ƶ����ʵ�������![]() ��̼���ƺ�̼�����ƻ����Һ�ʼ��ԣ�̼�������ˮ��̶ȴ���̼��������ӣ��ٽ�������غ��еøû����Һ������Ũ�ȴ�С˳���ǣ�
��̼���ƺ�̼�����ƻ����Һ�ʼ��ԣ�̼�������ˮ��̶ȴ���̼��������ӣ��ٽ�������غ��еøû����Һ������Ũ�ȴ�С˳���ǣ�![]() ��
��
![]() ����ˮϡ�ʹٽ�������룬����Һ�е������ӵ���Ŀ���ӣ���A����
����ˮϡ�ʹٽ�������룬����Һ�е������ӵ���Ŀ���ӣ���A����
B������ĵ���̶�������Һ��![]() ��С����B����
������B����
C����Һ�� ���¶Ȳ��䣬�����ƽ�ⳣ����ˮ�����ӻ��������䣬������Һ��
���¶Ȳ��䣬�����ƽ�ⳣ����ˮ�����ӻ��������䣬������Һ�� ���䣬��C��ȷ��
���䣬��C��ȷ��
D����Һ��ˮ�ĵ���̶������� ��С����D��ȷ��
��С����D��ȷ��
��ѡCD��
![]() ��Һ���������Ƶ����ʵ���Ϊ��
��Һ���������Ƶ����ʵ���Ϊ��![]() ��ϡ��������ʵ���Ϊ��
��ϡ��������ʵ���Ϊ��![]() ������Һ��Ϻ���Һ��ʾ���ԣ�������Ũ��Ϊ
������Һ��Ϻ���Һ��ʾ���ԣ�������Ũ��Ϊ![]() �����У�
������![]() �����
�����![]() ��
��![]() ��1��
��1��
![]() ���������Һ�����ԣ�����Һ��
���������Һ�����ԣ�����Һ��![]() ��������Һ��
��������Һ��![]()
![]() ����A��ȷ��
����A��ȷ��
B��������������ȣ��������ǿ�ᣬ������Һ�����ԣ�����������ᣬ������Һ�����ԣ���B����
C�������Һ�����ԣ�����Һ���������Һ���������ᣬ��Ũ�ȴ�����������Ũ�ȣ�����![]() ��һ������
��һ������![]() ����C����
����C����
D�������Һ�ʼ��ԣ�����Һ�����Ǽ������Һ��Ҳ����ֻ������Һ����![]() һ��С��
һ��С��![]() ����D��ȷ��
����D��ȷ��
��ѡAD��
![]() ��ͬŨ�ȵ�������Һ���������Խ�������������ˮ��̶�Խ����������ҺpHȷ�����ǿ������ͬŨ�ȵIJ�ͬ�ᣬ��ˮϡ�ʹٽ�������룬��ϡ����ͬ�ı������������Խ��������Һϡ������pH�仯ԽС������������Һ��pH֪��HCN��HClO��
��ͬŨ�ȵ�������Һ���������Խ�������������ˮ��̶�Խ����������ҺpHȷ�����ǿ������ͬŨ�ȵIJ�ͬ�ᣬ��ˮϡ�ʹٽ�������룬��ϡ����ͬ�ı������������Խ��������Һϡ������pH�仯ԽС������������Һ��pH֪��HCN��HClO��![]() ��
��![]() ����������Դ�С˳����
����������Դ�С˳����![]() ��������Һ��pH�仯��С����HCN����ѡA��
��������Һ��pH�仯��С����HCN����ѡA��
![]() ��HClO��
��HClO��![]() ��
��![]() ����������Դ�С˳����
����������Դ�С˳����![]() ������ǿ����ȡ����֪��
������ǿ����ȡ����֪��
A������![]() �����Զ��߷�ӦΪ
�����Զ��߷�ӦΪ![]() ����A��ȷ��
����A��ȷ��
B���������Դ���HCN�����Զ��߷�ӦΪ![]() ����B��ȷ��
����B��ȷ��
C��̼�����Դ��ڴ����ᣬ���Զ��߷�ӦΪ![]() ����C����
����C����
D������![]() ������
������![]() ��HCN���߲���Ӧ����D����
��HCN���߲���Ӧ����D����
��ѡAB��
![]() ��ʼ������pH��С������
��ʼ������pH��С������![]() �ȳ�����
�ȳ�����![]() ��
��![]() ��ʼ������
��ʼ������![]() �ֱ�Ϊ��
�ֱ�Ϊ��![]() ��
��![]() ������
������![]() ��
��

 ��1����Ԫ�¿�������ĩϵ�д�
��1����Ԫ�¿�������ĩϵ�д�����Ŀ��Ϊ�˲ⶨʵ���ҳ��ڴ�ŵ�Na2SO3����Ĵ��ȣ�ȷ��ȡM g������Ʒ�����250 mL��Һ���������������ʵ�鷽����
����I��ȡ50.00 mL������Һ�����������������ữ��BaCl2��Һ������I��ϴ�ӡ���������������õ�����������Ϊm1 g
������ȡ50.00 mL������Һ����a mol/L ������KMnO4��Һ���еζ���
ʵ��������¼���������±���
�ζ����� ʵ������ | 1 | 2 | 3 | 4 |
������Һ���/mL | 50.00 | 50.00 | 50.00 | 50.00 |
�ζ��ܳ�����/mL | 0.00 | 0.20 | 0.10 | 0.15 |
�ζ���ĩ����/mL | 20.95 | 21.20 | 20.15 | 21.20 |
(1)����250 mL Na2SO3��Һʱ�������õ���ʵ�������У��ձ����������ιܡ�ҩ��_______��________��
(2)����IΪ______________��������Ϊ______________��
(3)�ڷ������еζ��յ���жϷ�����_______________________________��
(4)�ڷ������з��������ӷ�Ӧ����ʽΪ____________________________��
(5)���ݷ��������ṩ�����ݣ�����Na2SO3�Ĵ���Ϊ___________����д�ɷ�����ʽ��
(6)��������������ԭ�ζ������У����´���ҺNa2SO3Ũ�ȱ�С����_____������ţ���
a���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ����ʼ���ӣ��ζ�����ʱ����
b���ü�ʽ�ζ�����ȡ50mL����Һ����ʱ��һ��ʼ�����ݣ��ζ�������û����
c����ʽ�ζ���������ˮ��ϴ��û��������KMnO4��Һ�����ϴ
d����ƿ������ˮ��ϴ��ֱ��װ50.00mL�Ĵ���Һ
e���ζ�����ʱ����ʼʱƽ�ӣ��ζ�����ʱ����
����Ŀ��![]() ��Ϊһ����Ҫ����ԭ�ϣ�������Ӧ���ڹ�ҵ�����������й����ʷ�Ӧ�Ĵ����о������������863�ƻ���
��Ϊһ����Ҫ����ԭ�ϣ�������Ӧ���ڹ�ҵ�����������й����ʷ�Ӧ�Ĵ����о������������863�ƻ���
(1)���������н�ǿ��ѡ���ԣ���רһ�ԡ���֪��
��ӦI��![]()
��ӦII��![]()
д��NO�ֽ�����![]() ��
��![]() ���Ȼ�ѧ����ʽ______��
���Ȼ�ѧ����ʽ______��
(2)�ں��º���װ���г���һ������![]() ��
��![]() ����ij�����������½��з�ӦI����ò�ͬʱ���
����ij�����������½��з�ӦI����ò�ͬʱ���![]() ��
��![]() ����Ũ�������
����Ũ�������
ʱ�� | 0 | 5 | 10 | 15 | 20 | 25 |
|
|
|
|
|
|
|
|
|
|
|
|
|
�������й���������ȷ����______��
A.ʹ�ô���ʱ���ɽ��÷�Ӧ�Ļ�ܣ��ӿ��䷴Ӧ����
B.�����������![]() ʱ��˵����Ӧ�Ѵ�ƽ��
ʱ��˵����Ӧ�Ѵ�ƽ��
C.��������![]() ʱ��˵����Ӧ�Ѵ�ƽ��
ʱ��˵����Ӧ�Ѵ�ƽ��
D.ǰ10�����ڵ�ƽ������![]()
![]()
(3)��������ʱ�ᷢ����������������ӦI��![]() Ϊ����ij�����Ը÷�Ӧ��ѡ���ԣ���1L�ܱ������г���1mol
Ϊ����ij�����Ը÷�Ӧ��ѡ���ԣ���1L�ܱ������г���1mol![]() ��2mol
��2mol![]() ������й����ʵ�����ϵ��ͼ1��
������й����ʵ�����ϵ��ͼ1��

�ٸô����ڵ���ʱѡ��Ӧ______![]() ����I������II��
����I������II��![]() ��
��
��![]() ʱ��
ʱ��![]() ��ƽ�ⳣ��
��ƽ�ⳣ��![]() ______
______![]() ��Ҫ��ó���������ֻ���г����ּ���ʽ
��Ҫ��ó���������ֻ���г����ּ���ʽ![]() ��
��
��![]() ���B����������NO�����ʵ����ٵ���Ҫԭ��______��
���B����������NO�����ʵ����ٵ���Ҫԭ��______��
(4)�ϳɰ���ҵ���������������![]() ����ҵ�ϳ��ø�Ũ�ȵ�
����ҵ�ϳ��ø�Ũ�ȵ�![]() ��Һ����
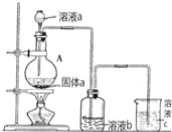
��Һ����![]() ������ҺX�������õ�ⷨʹ
������ҺX�������õ�ⷨʹ![]() ��Һ��������װ����ͼ2��ʾ��
��Һ��������װ����ͼ2��ʾ��
���������������ķ�Ӧ����______��![]() ��
��
�ڼ���![]() ��������������ԭ��______��
��������������ԭ��______��
(5)�Ʊ�����ʱ������һ���ķ�Һ����ҵ�ϳ�������������ͳ�ȥ��Һ�е�![]() ��
��
��֪��![]() ʱ��
ʱ��![]() ��
��![]() ��
��![]()
���ڼ�����Һ�е�����Ũ��ʱ���漰����ĵ���ͨ��Ҫ���н��ƴ�������![]() �������
�������![]() ______
______![]() ȡ��������
ȡ��������![]() ��
��
����֪��ij��Һ�ӽ������ԣ������������������ᣬ����Һ��![]() ______
______![]() ������������λ��Ч����
������������λ��Ч����![]() ���ͻ����CuS������
���ͻ����CuS������