��Ŀ����
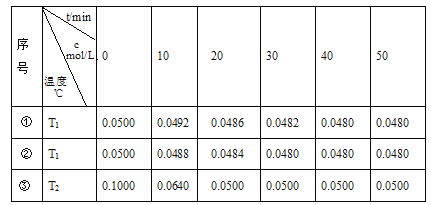
����Ŀ������Ԫ�صĻ��ϼ��Ʋ����ʵ������ǻ�ѧ�о�����Ҫ�ֶΡ���ͼ����Ԫ�صij������ϼ��벿���������Ķ�Ӧ��ϵ��
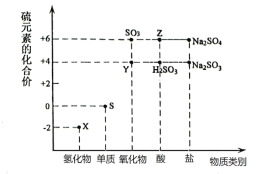
��1������Ԫ�ػ��ϼ۱仯�ĽǶȷ�����ͼ�м������������л�ԭ�ԵĻ�������___���ѧʽ����
��2����X��Y��ϣ������ɵ���ɫ���壬�÷�Ӧ�л�ԭ���������������ʵ���֮��Ϊ___��
��3����������Y�ķ�����___��
��4��Z��Ũ��Һ��ͭ������һ�������¿��Է�����ѧ��Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ___��
��5��Na2S2O3����Ҫ�Ļ���ԭ�ϡ���������ԭ��Ӧ�ĽǶȷ����������Ʊ�Na2S2O3�ķ��������Ͽ��е���___������ţ���
a.Na2S+S b.SO2+Na2SO4 c.Na2SO3+S d.Na2SO3+Na2SO4 e.SO2+ Na2S2+Na2CO3
��6����֪Na2SO3�ܱ�K2Cr2O7����ΪNa2SO4��24mL0.05 mol��L-1��Na2SO3��Һ��20mL0.02mol��L-1��K2Cr2O7��Һǡ����ȫ��Ӧʱ��CrԪ���ڻ�ԭ�����еĻ��ϼ�Ϊ___��
���𰸡�SO2��H2SO3��Na2SO3 2��1 ��Yͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ�����Ⱥ��ֱ��ɫ Cu+2H2SO4��Ũ��![]() CuSO4+2H2O+SO2�� ce +3
CuSO4+2H2O+SO2�� ce +3
��������
SԪ�صĻ��ϼ���-2�ۡ�0�ۡ�+4�ۡ�+6�ۣ�����ͼʾ��X��H2S��Y��SO2��Z��H2SO4��
��1�������м��̬��Ԫ�ؼ��л�ԭ�����������ԣ�
��2��XΪH2S����H2S��SO2��ϣ������ɵ���ɫ���壬�����ö�������������������������ɵ���ɫ����S��ˮ��Ԫ�ػ��ϼ۽��͵���������������Ԫ�ػ��ϼ����ߵ�Ϊ��ԭ����
��3�������������Ư���Կ���ʹƷ����Һ��ɫ��
��4��Ũ������Һ��ͭ�����ڼ��������¿��Է�����ѧ��Ӧ��������ͭ�����������ˮ��
��5��Na2S2O3��SΪ+2�ۣ���������ԭ�ĽǶȷ�������Ӧ����SԪ�ػ��ϼ۱���ֱ����2��С��2��
��6��Na2SO3������ΪNa2SO4��SԪ�ػ��ϼ���+4������Ϊ+6�ۣ�K2Cr2O7��CrԪ�ػ��ϼ�Ϊ+6��Cr������ԭ��Ӧ����CrԪ���ڲ����еĻ��ϼ�Ϊa�ۣ����ݵ���ת���غ����a��ֵ��
��1�������м��̬��Ԫ�ؼ��л�ԭ�����������ԣ�SԪ�صĻ��ϼ���-2�ۡ�0�ۡ�+4�ۡ�+6�ۣ�����0�ۺ�+4��S�Ļ�������л�ԭ�����������ԣ���SO2��H2SO3��Na2SO3��
��2����H2S��SO2��ϣ������ɵ���ɫ���壬�����ö�������������������������ɵ���ɫ����S��ˮ��2H2S+SO2=3S��+2H2O����Ӧ����Ԫ�ػ��ϼ�-2�۱仯Ϊ0�ۣ�H2S����ԭ����+4�۱仯Ϊ0�ۣ���������������������÷�Ӧ�Ļ�ԭ���������������ʵ���֮��Ϊ2��1��
��3�������������Ư���Կ���ʹƷ����Һ��ɫ�������ֻ�ָ���ɫ��������������ķ����ǣ�����������ͨ��Ʒ����Һ�У�Ʒ����Һ��ɫ�����Ⱥ��ֱ��ɫ��
��4��Ũ������Һ��ͭ�����ڼ��������¿��Է�����ѧ��Ӧ��������ͭ�����������ˮ����Ӧ�Ļ�ѧ����ʽΪ��Cu+2H2SO4��Ũ��![]() CuSO4+2H2O+SO2����
CuSO4+2H2O+SO2����
��5��Na2S2O3��SΪ+2�ۣ���������ԭ�ĽǶȷ�������Ӧ����SԪ�ػ��ϼ۱���ֱ����2��С��2��a��S���ϼ۶�С��2�� b��d��S�Ļ��ϼ۶�����2��c��e�������⣬��ѡc��e��
��6����CrԪ���ڲ����еĻ��ϼ�Ϊa�ۣ����ݵ���ת���غ㣬��24��10-3L��0.05mol/L����6-4��=20��10-3L��0.02mol/L��2����6-a�������a=+3��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�