��Ŀ����
CuSO4��5H2O��ͭ����Ҫ��������Ź㷺��Ӧ�á�������CuSO4��5H2O��ʵ�����Ʊ�����ͼ��
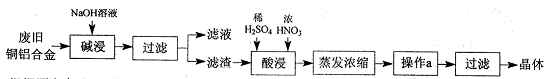
�����������������գ�
��1�����������Ŀ���� ��д���йص����ӷ���ʽ ��
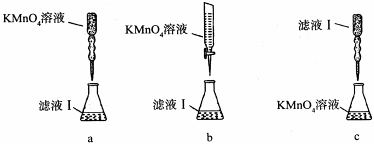
��2�����������ȼ�������ϡ���ᣬȻ���ٵμ�����Ũ���ᣬ�ڷ����ܽ�ʱ���Թ۲쵽��ʵ�������� ��
��3������a������Ϊ ���Ƶõ�CuSO4��5H2O�п��ܴ�������ͭ���ʣ���ȥ�������ʵ�ʵ���������Ϊ ��
��4����֪��CuSO4+2NaOH=Cu��OH��2��+ Na2SO4����ȡ0��26 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0��1000 mol/L����������Һ28��00 mL����Ӧ��ȫ����������������0��1000 mol/L����ζ����յ㣬��������8��00 mL�����������CuSO4��5H2O����������Ϊ �������ζ��У��ζ�����ע������֮ǰ����������ˮϴ�������� ��
��5���ڡ�������IJ����У�����ֻ����Ũ���ᣬд������ʱ�Ļ�ѧ����ʽ ��
������Ũ���ỻ�ɹ������⣬����ʱ������������ͭ��ָ�����ַ������ŵ� ��

�����������������գ�
��1�����������Ŀ���� ��д���йص����ӷ���ʽ ��
��2�����������ȼ�������ϡ���ᣬȻ���ٵμ�����Ũ���ᣬ�ڷ����ܽ�ʱ���Թ۲쵽��ʵ�������� ��
��3������a������Ϊ ���Ƶõ�CuSO4��5H2O�п��ܴ�������ͭ���ʣ���ȥ�������ʵ�ʵ���������Ϊ ��
��4����֪��CuSO4+2NaOH=Cu��OH��2��+ Na2SO4����ȡ0��26 g�ᴿ���CuSO4��5H2O��������ƿ�У�����0��1000 mol/L����������Һ28��00 mL����Ӧ��ȫ����������������0��1000 mol/L����ζ����յ㣬��������8��00 mL�����������CuSO4��5H2O����������Ϊ �������ζ��У��ζ�����ע������֮ǰ����������ˮϴ�������� ��
��5���ڡ�������IJ����У�����ֻ����Ũ���ᣬд������ʱ�Ļ�ѧ����ʽ ��
������Ũ���ỻ�ɹ������⣬����ʱ������������ͭ��ָ�����ַ������ŵ� ��
��1���ܽ����������ȥ���� 2Al+2H2O+2OH-=2AlO2-+3H2��
��2�������ܽ⣬��Һ����������ɫ�������������Һ�Ϸ���Ϊ����ɫ
��3����ȴ�ᾧ �ؽᾧ
��4��0.92 �ñ�������ϴ2~3��
��5��Cu+2H2SO4(Ũ)==CuSO4+SO2��+2H2O
�����������1��ʵ��Ŀ�������÷Ͼ���ͭ�Ͻ��Ʊ�����ͭ���壬��Ӧ�ȳ��ӡ�������dz��ӹ��̣���ȥ��������ۺ��ܽ�����������ӷ���ʽΪ2Al+2H2O+2OH-=2AlO2-+3H2������2����������Ũ�����ϡ�ͣ���ͭ��Ӧ����������ͭ��NO���壬������Ϊͭ�ܽ⣬��Һ��Ϊ��ɫ������ɫ���������ֻ��Һ�Ϸ���Ϊ����ɫ�����NO2������3��������ȴ�ᾧ�ķ�������������ͭ���壬��ȥ�����еĿ��������ʵķ������ؽᾧ��
��4����CuSO4���������Ƶ���n(NaOH)=28.00��0.1000��10-3��8.00��0.1000��10-3=2��10-3mol
n(CuSO4��5H2O) = n(NaOH)/2=10-3mol w(CuSO4��5H2O)=10-3��250��0.26=0.92
(5)����������һ����ɫ�����������Ũ������Բ�������Ⱦ�����壬����������

��ϰ��ϵ�д�
 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�
�����Ŀ
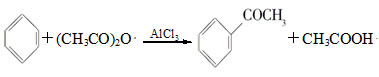
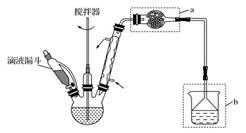
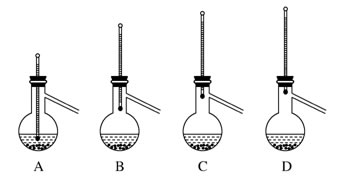
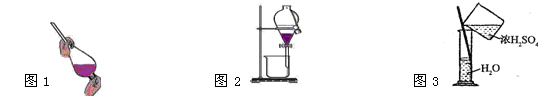
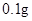
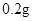 ��
�� /�ա�
/�ա�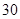 ��
��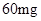 /�գ�������
/�գ�������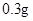 /��
/��
 12Na2CrO4+3Fe2O3+7KCl+12H2O��
12Na2CrO4+3Fe2O3+7KCl+12H2O��