��Ŀ����
ʵ�����Ʊ�����ͪ�Ļ�ѧ����ʽΪ��
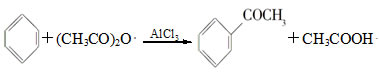
�Ʊ������л���CH3COOH��AlCl3�D��CH3COOAlCl2��HCl���ȸ���Ӧ��
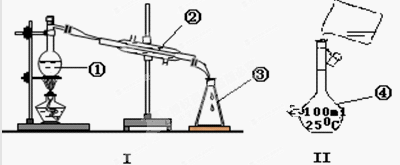
��Ҫʵ��װ�úͲ������£�
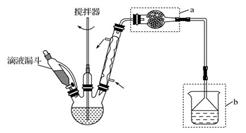
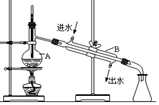
(��)�ϳɣ�������ƿ�м���20g��ˮ���Ȼ�����30mL
��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6mL
��������10mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ
�����������μ���Ϻ���Ȼ���1Сʱ��
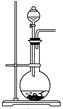
(��)�������ᴿ���ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л����ˮ���ñ���ȡ����Һ�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ������ֲ�Ʒ�õ�����ͪ
�ش��������⣺(1)����a�����ƣ�________��װ��b�����ã�________��
(2)�ϳɹ�����Ҫ����ˮ������������_______________________��
(3)�����������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���________��
(4)�������ᴿ�����ڵ�Ŀ����________���ò������Ƿ�ɸ����Ҵ���ȡ��________(��ǡ���)��ԭ����______________________��
(5)��Һ©��ʹ��ǰ��________��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ��________����Һ©����������̨����Ȧ�Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ��________��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ�����
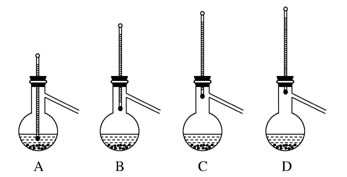
(6)�ֲ�Ʒ�����ᴿʱ������װ�����¶ȼ�λ����ȷ����________�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����________��

�Ʊ������л���CH3COOH��AlCl3�D��CH3COOAlCl2��HCl���ȸ���Ӧ��
��Ҫʵ��װ�úͲ������£�

(��)�ϳɣ�������ƿ�м���20g��ˮ���Ȼ�����30mL
��ˮ����Ϊ���ⷴӦҺ���¹��죬�߽���������μ�6mL
��������10mL��ˮ���Ļ��Һ�����Ƶμ����ʣ�ʹ��ӦҺ
�����������μ���Ϻ���Ȼ���1Сʱ��
(��)�������ᴿ���ٱ߽���������μ�һ����Ũ�������ˮ���Һ������õ��л����ˮ���ñ���ȡ����Һ�۽��٢������л���ϲ���ϴ�ӡ������ȥ�����õ�����ͪ�ֲ�Ʒ������ֲ�Ʒ�õ�����ͪ
�ش��������⣺(1)����a�����ƣ�________��װ��b�����ã�________��
(2)�ϳɹ�����Ҫ����ˮ������������_______________________��
(3)�����������ͱ��Ļ��Һһ���Ե�������ƿ�����ܵ���________��
| A����Ӧ̫���� | B��Һ��̫������� | C����Ӧ�仺�� | D������������ |
(5)��Һ©��ʹ��ǰ��________��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ��________����Һ©����������̨����Ȧ�Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ��________��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ�����
(6)�ֲ�Ʒ�����ᴿʱ������װ�����¶ȼ�λ����ȷ����________�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����________��

(1)���������HCl����
(2)��ֹ���Ȼ�����������ˮ��(ֻ�����Ȼ���ˮ���������ˮ��Ҳ��)
(3)AD
(4)���ܽ���ˮ�еı���ͪ��ȡ�����Լ�����ʧ���Ҵ���ˮ����
(5)��©�������Ͽڲ�����(��ʹ���ϵİ��۶�©�����ϵ�С��)
(6)CAB
(2)��ֹ���Ȼ�����������ˮ��(ֻ�����Ȼ���ˮ���������ˮ��Ҳ��)
(3)AD
(4)���ܽ���ˮ�еı���ͪ��ȡ�����Լ�����ʧ���Ҵ���ˮ����
(5)��©�������Ͽڲ�����(��ʹ���ϵİ��۶�©�����ϵ�С��)
(6)CAB
(1)����a�����ƣ�����ܣ�װ��b�����ã�����HCl���壻�𰸣����������HCl���塣(2)�ϳɹ�����Ҫ����ˮ������AlCl3��3H2O=Al(OH)3��3HCl,CH3COOCOCH2CH3��H2O��2CH3COOH,�𰸣���ֹ���Ȼ���ˮ���������ˮ�⣻��A����Ӧ��Ũ�ȴ�Ӧ���ʿ죬���·�Ӧ̫���ң�A�п��ܣ�C�����ܣ�B�����ս�ȫ�����룬��������ΪҺ��̫���������B�����ܣ�D��������CH3COOHŨ�ȴ���Ʒ���࣬D�п��ܣ�ѡAD����ˮ���ñ���ȡ����Һ�����ܽ���ˮ�еı���ͪ��ȡ�����Լ�����ʧ�����ܰѱ������Ҵ����Ҵ���ˮ���ܣ����ֲ㣻�𰸣����ܽ���ˮ�еı���ͪ��ȡ�����Լ�����ʧ���Ҵ���ˮ���ܣ��ɷ�Һ©��ʹ��ǰ���©��ϴ�����á���ȡʱ���Ⱥ�������ȡҺ����ȡ��������ҡ����������Һ©����������̨����Ȧ�Ͼ���Ƭ�̣��ֲ㡣�������²�Һ��ʱ��Ӧ�ȴ��Ͽڵ�����(��ʹ���ϵİ��۶�©�����ϵ�С��)��Ȼ������ų��²�Һ�壬�ϲ�Һ����Ͽڵ������𰸣���©�������Ͽڲ�����(��ʹ���ϵİ��۶�©�����ϵ�С��)��������ʱ���������¶ȣ�C��ȷ�����ܻᵼ���ռ����IJ�Ʒ�л��еͷе����ʵ�װ����AB��D�����ռ����е���ߵ���֡��𰸣�CAB��

��ϰ��ϵ�д�
�����Ŀ