��Ŀ����
12������֧�Թ��зֱ����������ͬ����ɫ��Һ�������²��������йز����������۵���������ȷ���ǣ�������| ���� | ���� | ���� | |
| A | �ȵμ�BaCl2��Һ�ٵμ�HCl��Һ | ���ɰ�ɫ���� | ԭ��Һ����SO42- |
| B | ��CCl4�������� | �ϲ���Һ����ɫ | ԭ��Һ����I2 |
| C | �μ�NaOH��Һ����ʪ�� ��ɫʯ����ֽ�����Թܿ� | ��ֽ������ | ԭ��Һ����NH4+ |
| D | �ýྻ��˿պȡ��Һ������ɫ��Ӧ | ����ʻ�ɫ | ԭ��Һ����Na+ |
| A�� | A�� | B�� | B�� | C�� | C�� | D�� | D�� |
���� A��̼������ӡ�����������ӡ���������ӡ������ӵȵμ�BaCl2��Һ���������ɰ�ɫ������
B�����Ȼ�̼���ܶȴ���ˮ��
C���μ�ϡ����������Һ�����ȣ���ֽ��������˵��ԭ��Һ����笠����ӣ�
D���Ƶ���ɫ��ӦΪ��ɫ��
��� �⣺A���еμ��Ȼ�����Һ���а�ɫ�������ɣ�����˵��ԭ��Һ������������ӣ���Ϊ̼������ӡ�����������ӡ���������ӡ������ӵȵμ��Ȼ�����Һ���������ɰ�ɫ��������A����
B�����Ȼ�̼�ѵ��ˮ��Һ����ȡ���������Ȼ�̼�ܶȱ�ˮ���²���Һ����ɫ����B����
C��������������ˮ������Һ�к���������NH4+ʱ���μ�ϡNaOH��Һ����ų�NH3��������Ҫ���ȣ���C����
D���Ƶ���ɫ��ӦΪ��ɫ���ýྻ��˿պȡ��Һ������ɫ��Ӧ������ʻ�ɫ����ԭ��Һ����Na+����D��ȷ��
��ѡD��
���� ���⿼�黯ѧʵ�鷽�������ۣ��漰��������ӡ��ⵥ�ʡ������Ӻ�笠����ӵļ��飬Ҫ���ճ������ӵļ��鷽����ע���ų��������ӵĸ��ţ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�����Ŀ
9�������£��ڢٴ�ˮ����pH=3�����ᣬ��pH=3��NH4Cl����pH=11��NaOH��Һ�У�ˮ�ĵ���ȴ�СΪ��������
| A�� | ��=��=��=�� | B�� | �ۣ���=�ܣ��� | C�� | �ۣ��٣���=�� | D�� | ��=�ڣ���=�� |
20��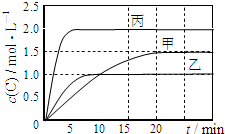 ��ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A��g��+xB��g��?2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����������ͼ��ʾ��
��ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A��g��+xB��g��?2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����������ͼ��ʾ��
����˵����ȷ���ǣ�������
 ��ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A��g��+xB��g��?2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����������ͼ��ʾ��
��ס��ҡ��������ܱ������г���һ������A��B��������Ӧ��A��g��+xB��g��?2C��g�����������ķ�Ӧ�¶ȡ���Ӧ����ʼ������Ӧ������C��Ũ����ʱ��仯��ϵ�ֱ����������ͼ��ʾ��| ���� | �� | �� | �� |
| �ݻ� | 0.5L | 0.5L | 1.0L |
| �¶�/�� | T1 | T2 | T2 |
| ��Ӧ�� ��ʼ�� | 1.5molA 0.5molB | 1.5molA 0.5molB | 6.0molA 2.0molB |
| A�� | x=1 | |
| B�� | 10min���������з�Ӧ��ƽ������v��B��=0.025mol•L-1•min-1 | |
| C�� | ��ͼ��֪��T1��T2���Ҹ÷�ӦΪ���ȷ�Ӧ | |
| D�� | T1�棬��ʼʱ�������г���0.5molA��1.5molB��ƽ��ʱA��ת����Ϊ75% |
17�� �����£���0.2mol•L-1 Al2��SO4��3��Һ�У���μ���1.0mol•L-1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����仯������ͼ�������й�˵��������ǣ�������
�����£���0.2mol•L-1 Al2��SO4��3��Һ�У���μ���1.0mol•L-1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����仯������ͼ�������й�˵��������ǣ�������
 �����£���0.2mol•L-1 Al2��SO4��3��Һ�У���μ���1.0mol•L-1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����仯������ͼ�������й�˵��������ǣ�������
�����£���0.2mol•L-1 Al2��SO4��3��Һ�У���μ���1.0mol•L-1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����仯������ͼ�������й�˵��������ǣ�������| A�� | a��ʱ����Һ�����Ե�ԭ���ǣ�Al3++3H2O?Al��OH��3��+3H+ | |
| B�� | b��c�Σ������NaOH��Ҫ��������Al��OH��3���� | |
| C�� | c�䡫d�Σ���Һ�Լ��Ե�ԭ���ǣ�NaOH�TNa++OH- | |
| D�� | d��e�Σ������ķ�ӦΪ��Al��OH��3+NaOH�TNaAlO2+2H2O |
4����NAΪ�����ӵ��곣����ֵ������������ȷ���ǣ�������
| A�� | ��״���£�33.6L�������к��з�ԭ�ӵ���ĿΪ1.5NA | |
| B�� | ���³�ѹ�£�7.0g��ϩ���ϩ�Ļ�����к���̼�������ĿΪNA | |
| C�� | 50 mL18.4mol/LŨ����������ͭ�ȷ�Ӧ������S02���ӵ���ĿΪ0.46NA | |
| D�� | ij�ܱ�����ʢ��0.1mol N2��0.3mol H2����һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0 6NA |
1��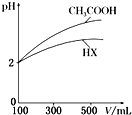 ˮ��������ԴȪ����ҵ��ѪҺ�����е���������ش��������⣺
ˮ��������ԴȪ����ҵ��ѪҺ�����е���������ش��������⣺
��1����ˮ��25��ʱ��pH=7�����¶���1mol•L-1��NaOH��Һ�У���ˮ�������
c��OH-��=10-14mol•L-1��
��2��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ�ⷽ��ʽΪC${O}_{3}^{2-}$+H2O?HC${O}_{3}^{-}$+OH-��HC${O}_{3}^{-}$+H2OH2CO3+OH-����ˮ�������c��OH-��=0.001mol•L-1��
��3�������Ϊ100mL��pH��Ϊ2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ��볣��С�ڣ�����ڡ���С�ڡ����ڡ���CH3COOH�ĵ��볣����������ϡ����ͬ������һԪ��HX��pH�仯����CH3COOH��С�������Խ��������볣����С��
��4�����볣���Ǻ���������ʵ���̶�ǿ��������������֪��
25��ʱ���е�Ũ�ȵ�NaCN��Һ��Na2CO3��Һ��CH3COONa��Һ������Һ��pH�ɴ�С��˳��ΪNa2CO3��Һ��NaCN��Һ��CH3COONa��Һ��
 ˮ��������ԴȪ����ҵ��ѪҺ�����е���������ش��������⣺
ˮ��������ԴȪ����ҵ��ѪҺ�����е���������ش��������⣺��1����ˮ��25��ʱ��pH=7�����¶���1mol•L-1��NaOH��Һ�У���ˮ�������
c��OH-��=10-14mol•L-1��
��2��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������̼���ƹ��壬�õ�pHΪ11����Һ����ˮ�ⷽ��ʽΪC${O}_{3}^{2-}$+H2O?HC${O}_{3}^{-}$+OH-��HC${O}_{3}^{-}$+H2OH2CO3+OH-����ˮ�������c��OH-��=0.001mol•L-1��
��3�������Ϊ100mL��pH��Ϊ2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ����ͼ��ʾ����HX�ĵ��볣��С�ڣ�����ڡ���С�ڡ����ڡ���CH3COOH�ĵ��볣����������ϡ����ͬ������һԪ��HX��pH�仯����CH3COOH��С�������Խ��������볣����С��
��4�����볣���Ǻ���������ʵ���̶�ǿ��������������֪��
| ��ѧʽ | ���볣����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
2������˵����ȷ���ǣ�������
| A�� | ��ⱥ��ʳ��ˮ���Ƶý����� | |
| B�� | ͨ����ѧ��Ӧ�Ӻ�ˮ�п���ȡ�Ȼ��ơ��塢������� | |
| C�� | Ϊ����ǿ���������Һ�������Կ��������ữ | |
| D�� | ClO2��һ���д̼�����ζ�Ļ���ɫ���壬������ɱ�������⣬���㷺���ڻ�����Ư�ס����� �ȷ��� |