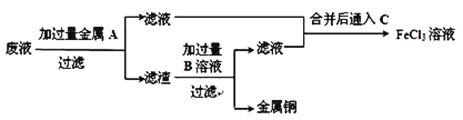
��Ŀ����
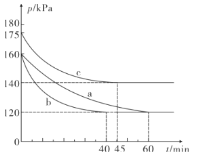
����Ŀ��������Ԫ�ص�ҡ������ˮ�к��д���±��Ԫ�ء�NaCl�����ṹʾ��ͼ������ʾ�������߳�Ϊa nm).

(1)Ԫ�� Na�ļ۵��ӱ����������ڸ��ܼ����γɵļ���̬Naԭ�ӣ���۵��ӹ����ʾʽΪ__________��
(2)���� NaCl��Cl Ԫ�ػ����γɶ��ּ�̬�Ļ������NaClO��NaClO2��NaClO3��NaClO4�����������ζ�Ӧ���������������ǿ���Խ���HClO4������ǿ��HClO3��ԭ��__________��
(3)���ʵ������£����NaClˮ��Һ���Ƶ� NaClO3��
��NaClˮ��Һ�д��ڵ�������������________������ţ���
A.���Ӽ� B.���Լ� C.��λ�� D.���
�ڸ��ݼ۲���ӶԻ������ۣ�Ԥ��ClO3-�Ŀռ乹��Ϊ________��д��һ��ClO3-�ĵȵ�����Ļ�ѧ���ţ�______________
(4)��NaCl�����У�Na λ��Cl ��Χ�ɵ���______��������ģ��ö�����ı߳���______nm��
(5)Na �뾶��Cl�뾶�ı�ֵΪ______������С�����3λ��![]() =1.414)��
=1.414)��
���𰸡�![]() HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2����(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ�� BCD ������ SO32-��IO3-��BrO3- ��
HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2����(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ�� BCD ������ SO32-��IO3-��BrO3- �� ![]() 0.414
0.414
��������
��1��Ԫ��Na����ɫ��Ӧ�ʻ�ɫ������̬Naԭ�Ӽ۵�����3s�ܼ�������3p�ܼ�����۵��ӹ����ʾʽΪ![]() ��
��
��2��HClO3��HClO4���ɷֱ��ʾΪ(HO)ClO2��(HO)ClO3��HClO3��ClΪ+5�ۣ�HClO4��ClΪ+7�ۡ����������Ը��ߣ�����H��O֮��ĵ��Ӷ���Oƫ�ƣ��������H+����HClO4�з��ǻ�������HClO3�࣬��HClO4���Ա�HClO3ǿ����
��3����A����NaCl��ˮ��Һ�У�NaCl����������ƶ��������Ӻ������ӣ����Ӽ����ƻ���A���������⣻
B��ˮ�����д��ڼ��Թ��ۼ���B�������⣻
C���������ṩ�չ����ˮ�����е���ԭ���ṩ�µ��Ӷԣ�������λ����C��ȷ��
D��ˮ���Ӽ���������D��ȷ��
��ѡBCD��
�ڸ��ݼ۲���ӶԻ������ۣ�ClO3-�ļ۲���Ӷ�����4������ԭ���ϵŵ��Ӷ�����1����ClO3-�Ŀռ乹��Ϊ�����Σ�ԭ�Ӹ�����ȣ��۵���������ͬ�ķ��ӻ����ӻ�Ϊ�ȵ����壬����ClO3-�ĵȵ������������SO32-��IO3-��BrO3-��
��4�������У���Na+Ϊ��������������ǰ��������6��Cl-��Na+λ��Cl-��Χ�ɵİ���������ģ���ͼ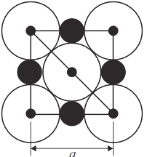 ���ö�����ı߳�=ͼ�жԽ��߳���һ��=
���ö�����ı߳�=ͼ�жԽ��߳���һ��=![]() ��
��
��5������ͼ ��NaCl�������������ӵ���̾���Ϊanm��һ�뼴
��NaCl�������������ӵ���̾���Ϊanm��һ�뼴![]() nm��Cl-�뾶Ϊ�Խ��ߵ�
nm��Cl-�뾶Ϊ�Խ��ߵ�![]() ����Ϊ
����Ϊ![]() nm����ͼ
nm����ͼ 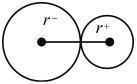 ��Na+�뾶Ϊ(
��Na+�뾶Ϊ(![]() nm-
nm-![]() nm)������Na+�뾶��Cl-�뾶֮��Ϊ(
nm)������Na+�뾶��Cl-�뾶֮��Ϊ(![]() nm-
nm-![]() nm)��
nm)��![]() nm=0.414 ��
nm=0.414 ��

 Сѧ��ĩ���100��ϵ�д�
Сѧ��ĩ���100��ϵ�д� ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д�