��Ŀ����
16��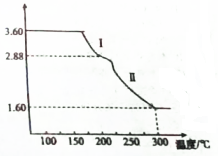 �ȼ��仯���������������������Ź㷺����;���ش��������⣺
�ȼ��仯���������������������Ź㷺����;���ش��������⣺��1�������ȵ�ͬ��Ԫ�أ����ȶ�3�����Ӳ㣬�������ڱ��е�λ��Ϊ�����ڣ��ڢ���A�壬HAt���ȶ��Ա�HCl�����ǿ������������
��2��Cl2��NaOH��Һ��Ӧ���¶�Ӱ��ϴ�����¶ȹ��ͣ�������NaClO���¶ȹ��ߣ�������NaClO3����NaOH��Һ��������Ӧʱ������Һ��ClO-��ClO3-�����ʵ���֮��Ϊ1��1����÷�Ӧ�����ӷ���ʽΪ4Cl2+8OH-=6Cl-+ClO-+ClO3-+4H2O��
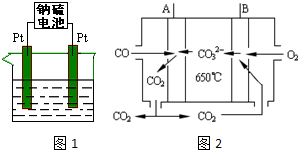
��3����ҵ�ϲ��õ�ⱥ��ʳ��ˮ�ķ�����ȡCl2���仯ѧ����ʽΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2������һ�������½�Cl2ͨ��NaOH��Һ�п��Ƶ�Ư��Һ��Ư��Һ����Ч�ɷ�ΪNaClO���ѧʽ������ͥʹ��Ư��Һʱ������ֱ�ӽӴ�������Ʒ����������Ʒ�������绯ѧ��ʴ�����������ĵ缫��ӦʽΪ2H2O+O2+4e-=4OH-��
��4�������NaCl��Һ�м����������NaClO3���ɼ���һ��������м�����ȳ�ȥ����Ӧ�Ļ�ѧ����ʽΪ3H2O+2Fe+NaClO3=NaCl+2Fe��OH��3�����õ������Ļ����ᆳһϵ��ת�����Եõ������������壨FeC2O4•2H2O������ȡ3.60g�����������壨��Է�������Ϊ180���������ط���������ȷֽ⣬�õ�ʣ�������������¶ȱ仯��������ͼ��ʾ��
�ٷ���ͼ�����ݣ�������Ϣд�����̢�����Ӧ�Ļ�ѧ����ʽFeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeC2O4+2H2O��
��300��ʱʣ�����ֻ��һ����ʵ�����������ͨ������ȷ����������Ļ�ѧʽFe2O3��
���� ��1�������ȵ�ͬ��Ԫ�أ���Ϊ�ڢ���AԪ�أ�Clλ�ڵ������ڣ��ǽ�����Cl��At���ǽ�����Խǿ���⻯��Խ�ȶ���
��2����NaOH��Һ��������Ӧʱ������Һ��ClO-��ClO3-�����ʵ���֮��Ϊ1��1��ʧȥ����Ϊ1����1-0��+1����5-0��=6����õ�����Ϊ6����Cl-��ClO-��ClO3-�����ʵ���֮��Ϊ6��1��1��
��3�����NaCl��Һ��Ӧ����������������NaOH��Cl2ͨ��NaOH��Һ�п��Ƶ�Ư��Һ������Ч�ɷ�ΪNaClO����������������Ʒ�������绯ѧ��ʴ�������������õ����ӣ�
��4��Fe��NaClO3����������ԭ��Ӧ��
�ٹ���Iʹ����������3.60-2.88g=0.72g��ǡ��Ϊ0.4molˮ��������
����Ԫ���غ������������FeԪ�ص�������������ԭ�Ӹ�����ȷ����ѧʽ��
��� �⣺��1�������ȵ�ͬ��Ԫ�أ���Ϊ�ڢ���AԪ�أ�Clλ�ڵ������ڣ�At���ȶ�3�����Ӳ㣬�������ڱ��е�λ��Ϊ�������ڵڢ���A�壬�ǽ�����Cl��At���ǽ�����Խǿ���⻯��Խ�ȶ�����֪HAt���ȶ��Ա�HCl����
�ʴ�Ϊ����������A������
��2����NaOH��Һ��������Ӧʱ������Һ��ClO-��ClO3-�����ʵ���֮��Ϊ1��1��ʧȥ����Ϊ1����1-0��+1����5-0��=6����õ�����Ϊ6����Cl-��ClO-��ClO3-�����ʵ���֮��Ϊ6��1��1�����ӷ�ӦΪ4Cl2+8OH-=6Cl-+ClO-+ClO3-+4H2O��
�ʴ�Ϊ��4Cl2+8OH-=6Cl-+ClO-+ClO3-+4H2O��
��3�����NaCl��Һ��Ӧ����������������NaOH����ӦΪ2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����Cl2ͨ��NaOH��Һ�п��Ƶ�Ư��Һ������Ч�ɷ�ΪNaClO����������������Ʒ�������绯ѧ��ʴ�������������õ����ӣ�������ӦΪ2H2O+O2+4e-=4OH-��
�ʴ�Ϊ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$2NaOH+H2��+Cl2����NaClO��2H2O+O2+4e-=4OH-��
��4�����������м��Ŀ���ǽ�NaClO3��ȫת��ΪNaCl��������мʱ������Ӧ�Ļ�ѧ����ʽΪ3H2O+2Fe+NaClO3=NaCl+2Fe��OH��3����
�ʴ�Ϊ��3H2O+2Fe+NaClO3=NaCl+2Fe��OH��3����
��3.60g�����������壬���ʵ���Ϊ0.2mol������Iʹ����������3.60-2.88g=0.72g��ǡ��Ϊ0.4molˮ������������̢����ķ�Ӧ�ǣ�����������������ʧȥ�ᾧˮ����Ӧ�Ļ�ѧ����ʽΪ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeC2O4+2H2O��
�ʴ�Ϊ��FeC2O4•2H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeC2O4+2H2O��
�ڲ������������е���Ԫ������Ϊ��3.6g��$\frac{56}{180}$��100%=1.12g���������������е���Ԫ����ȫת�����������У�
����������Ԫ�ص�����Ϊ��1.60g-1.12g=0.48g��
��Ԫ�غ���Ԫ�ص�������Ϊ��1.12g��0.48g=7��3��
������������Ļ�ѧʽΪFexOy��
��56x��16y=7��3��
x��y=2��3��
����������Ļ�ѧʽΪFe2O3��
�ʴ�Ϊ��Fe2O3��
���� ���⿼����ۺϣ��漰Ԫ�����ڱ���Ԫ�������ɡ�������ԭ��Ӧ���㼰���ӷ�Ӧ����⼰�缫��Ӧ�����ʺ����IJⶨ������ȣ�ע�ظ�Ƶ����Ŀ��飬���շ�Ӧԭ��Ϊ���Ĺؼ������ط�����Ӧ�á����������Ŀ��飬�ۺ��Խ�ǿ����Ŀ�Ѷ��еȣ�

| Ԫ�� | A | B | C | D |
| ���� �ṹ ��Ϣ | ��̬ԭ�Ӻ������������Ӳ㣬�������3��δ�ɶԵĵ��� | ��̬ԭ�ӵ�M����1�ԳɶԵ�p���� | ��̬ԭ�Ӻ�������Ų�Ϊ[Ar]3d104sx����+1��+2���ֳ������ϼ� | �����ֳ��������������һ����ұ��ҵ���õĻ�ԭ�� |
��2��AԪ�ص��⻯��ķе��ͬ��������Ԫ���⻯��е�ߣ���ߡ��͡�������ԭ��������֮����������
��3��DԪ�������������۵��ͬ��������Ԫ�������������۵�ͣ���ߡ��͡�������ԭ����CO2Ϊ���Ӿ��壬SiO2��ԭ�Ӿ��壮
��4����CԪ�ص���������Һ����μ������AԪ�ص��⻯��ˮ��Һ�������ɵ������Ļ�ѧʽΪ[Cu��NH3��4]SO4����������д��ڵĻ�ѧ��������ABD��������ĸ��
A�����Ӽ� B�����ۼ� C�������� D����λ�� E�����Ӽ�������
��5�����з��ӽṹͼ�еġ���ʾ�������Ԫ�ص�ԭ���г�ȥ�������ӵ�ʣ�ಿ�֣���O����ʾ��ԭ�ӣ�С�ڵ㡰•����ʾû���γɹ��ۼ����������ӣ����߱�ʾ���ۼ���
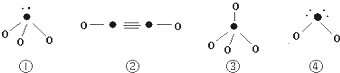
�������Ϸ����У�����ԭ�Ӳ���sp3�ӻ����Ǣ٢ۢܣ����ڼ��Է��ӵ��Ǣ٢ܣ������Ͼ���д��ţ����ڢڵķ�������3���Ҽ���2���м���
| A�� | ���ÿ�������������Ĺ���Ϊ�Ȼ������������� | |
| B�� | ��ˮ�е�������Br2��ʽ���ڣ����ÿ����������ɻ��Br2 | |
| C�� | ���չ����У�����SO2��Br2��ԭΪHBr������Cl2����HBr�õ�Br2 | |
| D�� | �Ȼ����̷����ķ�ӦΪ2Br-+Cl2�TBr2+2Cl- |

����˵����ȷ���ǣ�������
| A�� | �����л��ﶼ�Ƿ����� | |
| B�� | �����л��ﱽ���ϵ���ԭ��������ԭ��ȡ������һ�ȴ��ﶼֻ��2�� | |
| C�� | �������ʵ������������ʼ���NaOH��Һ�У���˾ƥ������NaOH��� | |
| D�� | 1 mol���ǻ���Ƥ�������Ժ�2 mol NaHCO3��Ӧ |