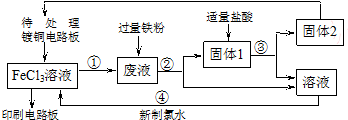
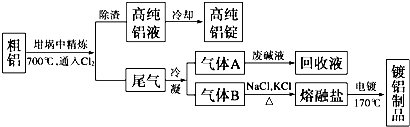
题目内容
6.铝是当前使用量仅次于铁的金属.(1)工业上以铝土矿(主要成分为Al2O3,含FeO、Fe2O3等杂质)为原料冶炼铝韵I艺流程如图1所示:

①溶浸时反应的离子方程式为Al2O3+2OH-=2AlO2-+H2O,提高溶浸时浸出速率的方法是(答出一条即可)铝土矿粉碎、适当加热、适当提高NaOH浓度等.
②溶液乙中溶质的化学式为NaHCO3,操作a为加热.
(2)如图2是工业冶炼铝的电解槽.
①工业上是用高熔点的A12O3而不是用低熔点的A1C13为原料制备铝的原因是氯化铝为共价化合物,熔融状态下不导电.
②冰晶石(Na3A1F6)的作用是降低氯化铝熔融温度,阴极上的电极反应式为Al3++3e-=Al.
③电解时会不断被消耗的电极是阳极(填“阴极”或“阳极,),若电解得到9kg铝,同时消耗石墨0.03kg,则电解过程中生成的气体在标准状况下的体积至少为5600 L.
(3)对铝制品进行抗腐蚀处理,可延长其使用寿命.以铝材为阳极、H2SO4溶液为电解质溶液进行电解,铝材表面形成氧化膜,阳极的电极反应式为2Al+3H2O-6e-=Al2O3+6H+.
分析 (1)铝土矿主要成分是Al2O3,含FeO、Fe2O3等杂质,将铝土矿用NaOH溶解,氧化铝溶解生成NaAlO2,FeO、Fe2O3不溶于NaOH溶液,然后采用过滤方法得到滤液甲为NaAlO2溶液,则试剂甲为NaOH;
向滤液中通入试剂二氧化碳,所以试剂乙为二氧化碳,得到Al(OH)3沉淀和NaHCO3,然后采用过滤方法得到固体甲为Al(OH)3,溶液乙为NaHCO3;将沉淀Al(OH)3解热分解得到Al2O3,电解熔融氧化铝得到Al单质;
①溶浸时氧化铝和NaOH溶液反应生成偏铝酸钠和水,提高溶浸时浸出速率的方法有:增大氢氧化钠溶液浓度、加热、增大反应物接触面积等;
②溶液乙中溶质的化学式为NaHCO3,将沉淀Al(OH)3解热分解得到Al2O3;
(2)①低熔点的A1C13为共价化合物,熔融状态下不导电;
②冰晶石(Na3A1F6)的作用是降低氧化铝熔融温度,阴极上铝离子得电子发生还原反应;
③电解时会不断被消耗的电极是阳极,若电解得到9kg铝,同时消耗石墨0.03kg,根据转移电子守恒计算生成氧气物质的量,得到气体包含二氧化碳和氧气,再根据V=nVm计算气体体积;
(3)阳极上铝失电子和水反应生成氧化铝和氢离子.
解答 解:(1)铝土矿主要成分是Al2O3,含FeO、Fe2O3等杂质,将铝土矿用NaOH溶解,氧化铝溶解生成NaAlO2,FeO、Fe2O3不溶于NaOH溶液,然后采用过滤方法得到滤液甲为NaAlO2溶液,则试剂甲为NaOH;
向滤液中通入试剂二氧化碳,所以试剂乙为二氧化碳,得到Al(OH)3沉淀和NaHCO3,然后采用过滤方法得到固体甲为Al(OH)3,溶液乙为NaHCO3;将沉淀Al(OH)3解热分解得到Al2O3,电解熔融氧化铝得到Al单质;
①溶浸时氧化铝和NaOH溶液反应生成偏铝酸钠和水,离子方程式为Al2O3+2OH-=2AlO2-+H2O,提高溶浸时浸出速率的方法有:增大氢氧化钠溶液浓度、加热、增大反应物接触面积等,所以可以采用将铝土矿粉碎、适当加热、适当提高NaOH浓度等,
故答案为:Al2O3+2OH-=2AlO2-+H2O;铝土矿粉碎、适当加热、适当提高NaOH浓度等;
②溶液乙中溶质的化学式为NaHCO3,将沉淀Al(OH)3解热分解得到Al2O3,故答案为:NaHCO3;加热;
(2)①低熔点的A1C13为共价化合物,熔融状态下以分子存在而不导电,故答案为:氯化铝是共价化合物,熔融状态下不导电;
②冰晶石(Na3A1F6)的作用是降低氧化铝熔融温度,阴极上铝离子得电子发生还原反应,电极反应式为Al3++3e-=Al,故答案为:降低氧化铝熔融温度;Al3++3e-=Al;
③电解时会不断被消耗的电极是阳极,若电解得到9kg铝,同时消耗石墨0.03kg,得到9000g铝转移电子物质的量=9000g27g/mol×39000g27g/mol×3=1000mol,当C被氧化生成CO时阳极得到气体体积最多,生成CO2时得到氧气体积最少,根据转移电子相等生成CO2时得到n(O2)=1000mol−30g12g/mol×441000mol−30g12g/mol×44=247.5mol,
根据C原子守恒得n(CO2)=30g12g/mol30g12g/mol=2.5mol,得到气体总体积=(247.5+2.5)mol×22.4L/mol=5600L,
故答案为:阳极;5600;
(3)阳极上铝失电子和水反应生成氧化铝和氢离子,电极反应式为2Al+3H2O-6e-=Al2O3+6H+,故答案为:2Al+3H2O-6e-=Al2O3+6H+.
点评 本题考查混合物的分离和提纯,为高频考点,涉及电解原理、金属冶炼、反应速率影响因素等知识点,明确反应原理及物质性质是解本题关键,难点是电极反应式的书写,易错点是(2)③的计算,注意阳极生成的气体不仅有氧气还有碳氧化物,注意氯化铝属于共价化合物,题目难度不大.

 名校课堂系列答案
名校课堂系列答案| A. | 铁跟氯化铁溶液反应:Fe+2Fe3+=3Fe2+ | |
| B. | 氨水跟盐酸反应:OH-+H+→H2O | |
| C. | 氢氧化钡跟稀硫酸反应:Ba2++OH-+H++SO42-→BaSO4↓+H2O | |
| D. | 碳酸钙跟盐酸反应:CO32-+2H+→CO2↑+H2O |
 一定温度下,向容积为2L的恒容密闭容器中充入6mol CO2和8mol H2,发生反应:CO2(g)+3H2(g)?CH3OH(g)+H2O(g)△H=-49.0kJ•mol-1,测得n(H2)随时间变化如曲线Ⅰ所示.下列说法正确的是( )
一定温度下,向容积为2L的恒容密闭容器中充入6mol CO2和8mol H2,发生反应:CO2(g)+3H2(g)?CH3OH(g)+H2O(g)△H=-49.0kJ•mol-1,测得n(H2)随时间变化如曲线Ⅰ所示.下列说法正确的是( )| A. | 该反应在0~8 min内CO2的平均反应速率是 0.375 mol•L-1•min-1 | |
| B. | 若起始时向上述容器中充入3 mol CO2和4 mol H2,则平衡时H2的体积分数大于20% | |
| C. | 若起始时向上述容器中充入4 mol CO2、2 mol H2、2 mol CH3OH和1mol H2O(g),则此时反应向正反应方向进行 | |
| D. | 改变条件得到曲线Ⅱ、Ⅲ,则曲线Ⅱ、Ⅲ改变的条件分别是升高温度、充入氦气 |
| A. | 标准状况下,11.2LSO3所含的原子数为1.5NA | |
| B. | 常温常压下,1.8g H2O中含有的电子数为0.8NA | |
| C. | 常温常压下,48g O2和O3的混合物中含有的氧原子数为3NA | |
| D. | 标准状况下,0.1mol Cl2与足量NaOH溶液反应时,转移的电子数为0.2NA |
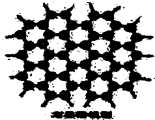 石墨晶体是层状结构,在每一层里,每一个碳原子都跟其它3个碳原子相结合.如图是石墨的晶体结构俯视图,图中每个黑点表示1个碳原子,而两黑点间的连线表示1个共价键,则石墨晶体中碳原子个数与共价键个数之比为( )
石墨晶体是层状结构,在每一层里,每一个碳原子都跟其它3个碳原子相结合.如图是石墨的晶体结构俯视图,图中每个黑点表示1个碳原子,而两黑点间的连线表示1个共价键,则石墨晶体中碳原子个数与共价键个数之比为( )| A. | 1:3 | B. | 2:3 | C. | 2:1 | D. | 3:2 |
| A. | 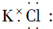 | B. |  | ||
| C. | 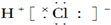 | D. | 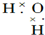 |