��Ŀ����
14������ѧƽ���ƶ�ԭ����ͬ��Ҳ����������ƽ����1����֪�ڰ�ˮ�д�������ƽ�⣺NH3+H2O?NH3•H2O?NH+OH-
��ˮ�м���MgCl2����ʱ��ƽ�������ƶ���OH-��Ũ�ȼ�С
��Ũ��ˮ�м�������NaOH���壬ƽ�������ƶ�����ʱ�������������д̼������������
��2���Ȼ���ˮ������ӷ���ʽΪFe3++3H2O?Fe��OH��3+3H+�����Ȼ�����Һ�м���̼��Ʒ�ĩ������̼������ܽ⣬��������ɫ���壬�����ӷ���ʽΪCaCO3+2H+�TCa2++H2O+CO2����ͬʱ�к��ɫ�������ɣ���ԭ����̼�������H+���ٽ��Ȼ�����ˮ�⣬ʹˮ����� Fe��OH��3�������ɣ��γɺ��ɫ����
��3����Mg��OH��2������Һ�м���NH4Cl��Һ��������Һ���ܽ⣬ԭ��Ϊ��Һ�д���ƽ�� Mg��OH��2 ��S��?Mg2+��aq��+2OH-��aq��������NH4Cl��Һ�ᷢ��OH-+NH4+=NH3•H2O������ƽ�����ܽⷽ���ƶ���������Һ���ܽ�
����ij��Ԫ�� H2A �ĵ��뷽��ʽ�ǣ�H2A=H++HA��HA-?A2-+H+���ش��������⣺
��1��H2A��ǿ����ʣ��ǿ����ʡ���������ʡ��ǵ���ʡ���
��2��Na2A ��Һ�Լ��ԣ�����ԡ��������ԡ������ԡ�����������A2-+H2O?HA-+OH-�������ӷ���ʽ��ʾ����
��3��NaHA ��Һ�����ԣ�����ԡ��������ԡ������ԡ�����������HA-?A2-+H+�������ӷ���ʽ��ʾ����
��4���� 0.1mol•L-1NaHA ��Һ�� pH=2���� 0.1mol•L-1 H2A��Һ�������ӵ����ʵ���Ũ�ȿ��ܣ�0.11mol•L�����������������=�����������ǣ���H2A��1�����������H+����HA-�ĵ��룻
��5��0.1mol•L NaHA��Һ�и�����Ũ���ɴ�С��˳����c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
���� ����1����ˮ�д�������ƽ�⣺NH3+H2O?NH3•H2O?NH4++OH-����������ܺ�笠����ӻ����������ӷ�Ӧ�����ʣ�ƽ�������뷽���ƶ����������������к���笠����ӻ����������ӣ�ƽ�����淴Ӧ�����ƶ����ݴ˷������
��2���Ȼ�����Һ��������ˮ�������������������ᣬ����̼��ƻ������������ɶ�����̼���壬�ٽ��Ȼ���ˮ��̶�����������������������
��3����Һ�д���ƽ�� Mg��OH��2 ��S��?Mg2+��aq��+2OH-��aq��������NH4Cl��Һ�ᷢ��OH-+NH4+=NH3•H2O������ƽ�����ܽⷽ���ƶ���������Һ���ܽ⣻
����1����H2A=H++HA-��֪��һ����ȫ���룬Ϊǿ����ʣ�
��2����HA-?H++A2-��֪��Na2AΪǿ�������Σ�
��3����һ����ȫ���룬�ڶ������ֵ��룬��Na2A��ҺӦ�ʼ��ԣ�NaHA��Һ����Ҫ����HA-�ĵ��룬����ҺӦ�����ԣ�
��4��0.1mol•L-1H2A��Һ��H2A�TH++HA-�������0.1mol/LH+��0.1mol•L-1NaHA��Һ��pH=2������HA-?H++A2-��֪�������0.01mol/LH+������һ���������ɵ�H+������HA-�ĵ��룻
��5��NaHA��Һ�����ԣ�����HA-?H++A2-����c��Na+����c��HA-����c��H+����c��OH-�������ˮ�ĵ��������
��� �⣺����1���ڰ�ˮ�д�������ƽ�⣺NH3+H2O?NH3•H2O?NH4++OH- ��ˮ�м���MgCl2����ʱ���ܽ����ɵ�þ���ӽ����������������������þ����������ƽ�������ƶ�������������Ũ�ȼ�С����Ũ��ˮ�м�������NaOH���壬�ܽ������������Ũ������ƽ�����ƣ����ɴ̼�����ζ�İ�����
�ʴ�Ϊ���ң���С�����д̼������������
�� 2���Ȼ�����Һ��������ˮ�������������������ᣬˮ������ӷ���ʽΪ��Fe3++3H2O?Fe��OH��3+3H+������̼��ƻ������������ɶ�����̼���壬��Ӧ�����ӷ���ʽΪ��CaCO3+2H+�TCa2++H2O+CO2������Ӧ�ٽ��Ȼ���ˮ��̶��������ɺ��ɫ��������������
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��CaCO3+2H+�TCa2++H2O+CO2����̼�������H+���ٽ��Ȼ�����ˮ�⣬ʹˮ����� Fe��OH��3�������ɣ��γɺ��ɫ������
��3��Mg��OH��2������Һ���д���ƽ�� Mg��OH��2 ��S��?Mg2+��aq��+2OH-��aq��������NH4Cl��Һ�ᷢ��OH-+NH4+=NH3•H2O������ƽ�����ܽⷽ���ƶ���������Һ���ܽ⣬���Կ���������Ϊ��Һ���ܽ⣬
�ʴ�Ϊ����Һ���ܽ⣻��Һ�д���ƽ�� Mg��OH��2 ��S��?Mg2+��aq��+2OH-��aq��������NH4Cl��Һ�ᷢ��OH-+NH4+=NH3•H2O������ƽ�����ܽⷽ���ƶ���������Һ���ܽ⣻
����1����H2A=H++HA-��֪��һ����ȫ���룬Ϊǿ����ʣ��ʴ�Ϊ��ǿ����ʣ�
��2����HA-?H++A2-��֪��Na2AΪǿ�������Σ�A2-ˮ���Լ��ԣ�ˮ�����ӷ�ӦΪA2-+H2O?HA-+OH-���ʴ�Ϊ�����ԣ�A2-+H2O?HA-+OH-��
��3����һ����ȫ���룬��NaHA��Һ����Ҫ����HA-�ĵ��룬����HA-?A2-+H+����Һ�����ԣ��ʴ�Ϊ�����ԣ�HA-?A2-+H+��
��4��0.1mol•L-1H2A��Һ��H2A�TH++HA-�������0.1mol/LH+��0.1mol•L-1NaHA��Һ��pH=2������HA-?H++A2-��֪�������0.01mol/LH+������һ���������ɵ�H+������HA-�ĵ��룬������Һ�������ӵ����ʵ���Ũ��С��0.1mol/L+0.01mol/L=0.11mol•L-1��
�ʴ�Ϊ��������H2A��1�����������H+����HA-�ĵ��룻
��5��NaHA��Һ�����ԣ�����HA-?H++A2-����c��Na+����c��HA-����c��H+����c��OH-�������ˮ�ĵ���H2O?H++OH-����c��H+����c��A2-������NaHA��Һ�и�������Ũ���ɴ�С��˳��Ϊc��Na+����c��HA-����c��H+����c��A2-����c��OH-����
�ʴ�Ϊ��c��Na+����c��HA-����c��H+����c��A2-����c��OH-����
���� ���⿼���˻�ѧƽ���Ӱ�������Լ�����ˮ��ķ����жϣ�ƽ���ƶ�ԭ����Ӧ�ã������������ʺ�ƽ���ƶ������ǽ���ؼ���ע���Ԫ������������ص㣬��Ŀ�Ѷ��еȣ�

 ��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�
��ͼͼ�麮����ҵ������ҵ���ִ�ѧ������ϵ�д�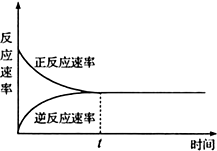
| A�� | tʱ�̱�ʾ�ڸ��������¸÷�Ӧ�ڴﵽ����� | |
| B�� | tʱ�̱�ʾ�÷�Ӧ�ﵽ��ѧƽ��״̬ | |
| C�� | tʱ����ǰ����Ӧ���ʴ����淴Ӧ���� | |
| D�� | tʱ�̼��Ժ�����Ӧ���ʵ����淴Ӧ���ʵ���0 |
| A�� | 22.4 L | B�� | 11.2 L | C�� | 2 L | D�� | 2.5L |
| A�� | ����[KAl��SO4��2•12H2O]��ˮ�����γ�Al��OH��3�����������ˮ�� | |
| B�� | ��1molCu����2molH2SO4��Ũ�����м��ȣ����к��ߵ����ʣ��ڷ�Ӧ���ܱ���ȫ���� | |
| C�� | ŨH2SO4��ǿ�����ԣ�����������Cu�������ҷ�Ӧ | |
| D�� | ��SO2ͨ����ˮ����ˮ��ɫ������ָܻ�ԭɫ |
| A�� | 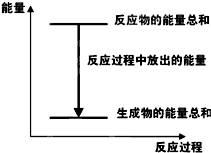 ��Ӧ������������ϵ������ͼ��ʾ | |
| B�� | �����÷�Ӧ��Ƴ�ԭ�����пΪ���� | |
| C�� | ��H��ֵ�뷴Ӧ����ʽ�Ļ�ѧ�������й� | |
| D�� | ���������Ϊԭ��أ�����32.5gп�ܽ�ʱ��ת�Ƶ�����Ϊ2NA |
| A�� | ����������ǡ���������ά�ؾ���Ϊͬ���칹�� | |
| B�� | CH3-CH=CH-CH3��C3H6һ����Ϊͬϵ�� | |
| C�� | ���顢�����Ҵ��������������Է���ȡ����Ӧ | |
| D�� | C3H8�Ķ��ȴ��ﹲ��3�� |
| A�� | -NO2��NO2 | B�� | HCOOCH3��CH3OCHO | C�� | HCOO-��-COOH | D�� | C2H5OH��CH3OCH3 |
��1��������������˵��������Ӧ�Ѵ�ƽ�����AC��
A�������������ƽ��Ħ���������ֲ���
B��2v��H2����=v��CH3OH����
C�������������ѹǿ���ֲ���
D����λʱ��������nmol CO��ͬʱ����2nmol H2
��2�����ݻ��̶��ĺ����ܱ������г���CO��H2����������Ӧ����Ӧ�ڵ�4minʱ��ﵽ���ȣ���ʱ������ѹǿ�뷴Ӧǰ֮��Ϊ3��5�����������ʵĸ������ʵ���Ũ�����±���
| ʱ��/Ũ�� | c��CO����mol/L�� | c��H2����mol/L�� | c��CH3OH����mol/L�� |
| ��ʼ | 0.200 | 0.300 | 0.000 |
| ��4min | a | b | c |
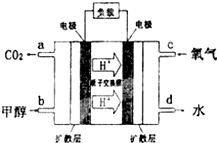
��3���״�-����ȼ�ϵ�أ�DMFC����һ�ָ�Ч�ܡ����� Ⱦ�綯�����ij��ص�أ��乤��ԭ����ͼ��ʾ����ȼ�ϵ�صĵ�ط�ӦʽΪ2CH3OH��g��+3O2��g���T2CO2��g��+4H2O��l�������ĵ缫��ӦʽΪCH3OH-6e-+H2O=CO2+6H+������õ�ع���ʱ��·��ͨ��1.2mol���ӣ�������CH3OH��0.2mol��
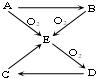 ��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�
��֪A��B��C��D��E������������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�P��Ӧ����δ�г���������ʱ��Ҫ�������������裩�������������о�����AԪ�أ�