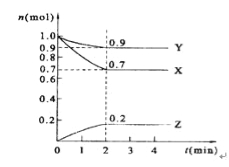
ЬтФПФкШн
ЁОЬтФПЁПНЋV1 mL 1.0 molЁЄL-1 HClШмвККЭV2 mLЮДжЊХЈЖШЕФNaOHШмвКЛьКЯОљдШКѓЃЌВтСПВЂМЧТМШмвКЕФЮТЖШЃЌЪЕбщНсЙћШчЯТЭМЫљЪОЃЌЪЕбщжаЪМжеБЃГжV1+V2=50ЁЃ

ЃЈ1ЃЉЖдгкЗДгІЃКHCl+NaOH= NaCl+H2OЃЌЗДгІЮяЫљОпгаЕФзмФмСПE1КЭЩњГЩЮяЫљОпгаЕФзмФмСПE2ЕФЙиЯЕЮЊЃКE1____E2ЁЃЃЈЬюЁА>ЁБЁЂЁА <ЁБЛђЁА=ЁБЃЉ
ЃЈ2ЃЉЪЕбщжаЫљгУNaOHШмвКЕФХЈЖШЮЊ____molЁЄL-1ЁЃ
ЃЈ3ЃЉШєКЌга8.0 g NaOHЕФЯЁШмвКгыЩдЙ§СПЕФ1 L 0.21 molЁЄL-1ЕФбЮЫсЗДгІЗХГі11.46 kJЕФШШСПЃЌдђБэЪОжаКЭШШЕФШШЛЏбЇЗНГЬЪНЮЊ___________________ЁЃ
ЃЈ4ЃЉШєШЁ50mL0.50mol/LбЮЫсгы50mL0.55mol/LNaOHШмвКжУгкШчЭМЫљЪОЕФзАжУжаНјаажаКЭШШЕФВтЖЈЪЕбщЃЌЛиД№ЯТСаЮЪЬтЃК
ЂйДгЪЕбщзАжУПДЃЌЦфжаЩаШБЩйЕФвЛжжВЃСЇгУЦЗЪЧ_________ЃЛ
ЪЕбщађКХ | Ц№ЪМЮТЖШt1/Ёц | жежЙЮТЖШ ЃЈt2ЃЉЁц | ||
бЮЫс | NaOHШмвК | ЦНОљжЕ | ||
1 | 25.1 | 24.9 | 25.0 | 28.0 |
2 | 25.1 | 25.1 | 25.1 | 28.2 |
3 | 25.1 | 25.1 | 25.1 | 28.3 |
4 | 25.1 | 25.1 | 25.1 | 27.4 |
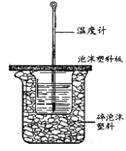
вбжЊбЮЫсЁЂNaOHШмвКУмЖШНќЫЦЮЊ1.00g/cm3ЃЌжаКЭКѓЛьКЯвКЕФБШШШШнc=4.18kJ/(kgЁЄK)ЃЌИљОнЩЯБэЪ§ОнЃЌЧѓГіИУЗДгІЕФжаКЭШШЁїH=____________ (БЃСє2ЮЛаЁЪ§) ЃЛ
ЂлЩЯЪіЪЕбщЪ§жЕНсЙћгы57.3 kJ/molгаЦЋВюЃЌВњЩњЦЋВюЕФдвђПЩФмЪЧ_______________ЁЃ
ЁОД№АИЁП > 1.5 HCl(aq)+NaOH(aq)===NaCl(aq)+H2O(l)ЁЁІЄH=-57.3 kJ/mol ЛЗаЮВЃСЇНСАшАє -51.83 kJ/mol ЪЕбщзАжУБЃЮТЁЂИєШШаЇЙћВюЃЛЗжЖрДЮЕЙШыNaOHШмвКЃЛЛьКЯШмвКЮДГфЗжНСАшЕШ
ЁОНтЮіЁПЪдЬтЗжЮіЃКБОЬтПМВщжаКЭШШШШЛЏбЇЗНГЬЪНЕФЪщаДЃЌжаКЭШШЕФВтЖЈКЭЮѓВюЗжЮіЁЃ
ЃЈ1ЃЉЗДгІHCl+NaOH= NaCl+H2OЪЧЗХШШЗДгІЃЌЗДгІЮяЫљОпгаЕФзмФмСПE1ДѓгкЩњГЩЮяЫљОпгаЕФзмФмСПE2ЁЃ
ЃЈ2ЃЉЭМаЮжаЮТЖШзюИпЪБЫсМюЧЁКУЭъШЋЗДгІЃЌV1=30mLЃЌдђV2=50mL-30mL=20mLЃЌnЃЈNaOHЃЉ=nЃЈHClЃЉЃЌcЃЈNaOHЃЉ![]() 20mL=1.0mol/L
20mL=1.0mol/L![]() 30mLЃЌcЃЈNaOHЃЉ=1.5mol/LЁЃ
30mLЃЌcЃЈNaOHЃЉ=1.5mol/LЁЃ
ЃЈ3ЃЉnЃЈNaOHЃЉ=8.0g![]() 40g/mol=0.2molЃЌЗДгІЩњГЩЫЎЕФЮяжЪЕФСПЮЊ0.2molЃЌдђжаКЭШШІЄH=-
40g/mol=0.2molЃЌЗДгІЩњГЩЫЎЕФЮяжЪЕФСПЮЊ0.2molЃЌдђжаКЭШШІЄH=-![]() =-57.3kJ/molЃЌжаКЭШШБэЪОЕФШШЛЏбЇЗНГЬЪНЮЊHCl(aq)+NaOH(aq)=NaCl(aq)+H2O(l)ЁЁІЄH=-57.3 kJ/molЁЃ
=-57.3kJ/molЃЌжаКЭШШБэЪОЕФШШЛЏбЇЗНГЬЪНЮЊHCl(aq)+NaOH(aq)=NaCl(aq)+H2O(l)ЁЁІЄH=-57.3 kJ/molЁЃ
ЃЈ4ЃЉЂйДгЪЕбщзАжУЩЯЙлВьЃЌШБЩйЕФвЛжжВЃСЇгУЦЗЪЧЛЗаЮВЃСЇНСАшАєЁЃ
Ђк50mL0.50mol/LбЮЫсгы50mL0.55mol/LNaOHШмвКЗДгІКѓNaOHЙ§СПЃЌЩњГЩЕФnЃЈH2OЃЉ=nЃЈHClЃЉ=0.50mol/L![]() 0.05L=0.025molЃЛЪЕбщ1ЁЂ2ЁЂ3ЁЂ4жежЙЮТЖШгыЦ№ЪМЮТЖШЕФВюжЕвРДЮЮЊ3.0ЁцЁЂ3.1ЁцЁЂ3.2ЁцЁЂ2.3ЁцЃЌгЩгкЪЕбщ4ЮѓВюНЯДѓЃЌЫљвдЪЕбщ1ЁЂ2ЁЂ3Ш§ДЮЪЕбщЮТЖШВюЕФЦНОљжЕЮЊ
0.05L=0.025molЃЛЪЕбщ1ЁЂ2ЁЂ3ЁЂ4жежЙЮТЖШгыЦ№ЪМЮТЖШЕФВюжЕвРДЮЮЊ3.0ЁцЁЂ3.1ЁцЁЂ3.2ЁцЁЂ2.3ЁцЃЌгЩгкЪЕбщ4ЮѓВюНЯДѓЃЌЫљвдЪЕбщ1ЁЂ2ЁЂ3Ш§ДЮЪЕбщЮТЖШВюЕФЦНОљжЕЮЊ![]() =3.1ЁцЃЌЗДгІЙ§ГЬжаЗХГіЕФШШСПQ=cmІЄt=4.18kJ/(kgЁЄK)
=3.1ЁцЃЌЗДгІЙ§ГЬжаЗХГіЕФШШСПQ=cmІЄt=4.18kJ/(kgЁЄK) ![]() ЃЈ1.00g/cm3
ЃЈ1.00g/cm3![]() 50mL+1.00g/cm3
50mL+1.00g/cm3![]() 50mLЃЉ
50mLЃЉ![]() 10-3kg/g
10-3kg/g![]() 3.1K=1.2958kJЃЌЩњГЩ1molH2OЗХГіЕФШШСПЮЊ1.2958kJ
3.1K=1.2958kJЃЌЩњГЩ1molH2OЗХГіЕФШШСПЮЊ1.2958kJ![]() 0.025mol=51.83kJ/molЃЌИУЗДгІЕФжаКЭШШІЄH=-51.83kJ/molЁЃ
0.025mol=51.83kJ/molЃЌИУЗДгІЕФжаКЭШШІЄH=-51.83kJ/molЁЃ
Ђл51.83kJ/mol![]() 57.3kJ/molЃЌЫЕУїВтЕУЕФжаКЭШШЕФЪЕбщЪ§жЕЦЋаЁЃЌПЩФмЪЧЪЕбщЙ§ГЬжаШШСПЩЂЪЇЫљжТЃЌВњЩњЦЋВюЕФПЩФмдвђЪЧЪЕбщзАжУБЃЮТЁЂИєШШаЇЙћВюЃЛЗжЖрДЮЕЙШыNaOHШмвКЃЛЛьКЯШмвКЮДГфЗжНСАшЕШЁЃ
57.3kJ/molЃЌЫЕУїВтЕУЕФжаКЭШШЕФЪЕбщЪ§жЕЦЋаЁЃЌПЩФмЪЧЪЕбщЙ§ГЬжаШШСПЩЂЪЇЫљжТЃЌВњЩњЦЋВюЕФПЩФмдвђЪЧЪЕбщзАжУБЃЮТЁЂИєШШаЇЙћВюЃЛЗжЖрДЮЕЙШыNaOHШмвКЃЛЛьКЯШмвКЮДГфЗжНСАшЕШЁЃ