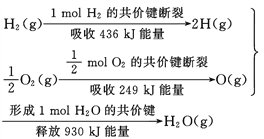
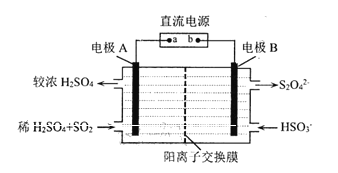
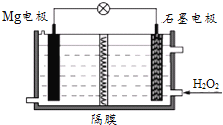
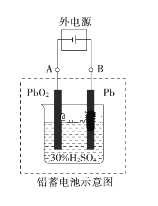
��Ŀ����
����Ŀ����.��֪ij���淴Ӧ��aA(g)��bB(g) ![]() cC(g)��dD(g)����ش��������⣺
cC(g)��dD(g)����ش��������⣺
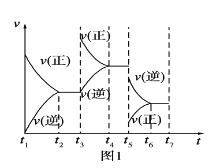
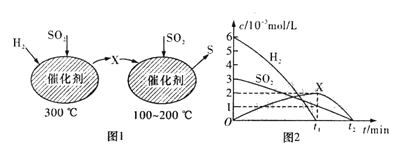
(1)����ʵ��ⶨ����������˸÷�Ӧ�ﵽƽ��״̬ʱ���ı����������Ӧ������ʱ��Ĺ�ϵͼ��(��ͼ1��ʾ)�����������D�����ı仯������ͼ���е�______�Σ�����ñ仯���̵����������_________________________________________��
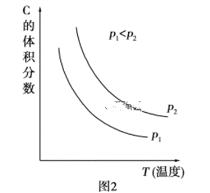
(2)����ʵ��ⶨ���������ͼ2���ɴ�ͼ����Եó��������¶ȣ�����ƽ�⽫��____(��������Ӧ�������淴Ӧ��)�����ƶ�����Ӧ������֮��Ĺ�ϵ��a��b______c��d(��������������С��������������������ȷ����)��
��.��ҵ�ϳɰ���Ӧ���£�N2��3H2![]() 2NH3����ش��������⣺
2NH3����ش��������⣺
(1)��һ��������2L���ܱ������н���ʵ�飬������������ݣ�
���� | N2 | H2 | NH3 |
��ʼ(mol) | 2 | 7 | 0 |
10s(mol) | 1.6 | ||
ƽ��ʱ(mol) | 2 |
��10s�ڣ���������ʾ�Ļ�ѧ��Ӧ������______���÷�Ӧ�Ļ�ѧƽ�ⳣ����________��
(2)���ݻ��������ɷֵ��������ʣ�����Ϊ��ҵ�Ͻ������ӻ�������з�������Ĵ�ʩ��________���Ӷ��ﵽ��Ӧ����ѭ�����õ�Ŀ�ġ�ʵ���Ҽ��鰱���ķ�����__________��
���𰸡�t3��t4 ���ӷ�Ӧ���Ũ�Ȼ�����AŨ�Ȼ�����BŨ�� �淴Ӧ ���� 0.12mol/(L��s) 0.25 ����ʹNH3��ΪҺ�壬��N2��H2���뿪 ��ʪ��ĺ�ɫʯ����ֽ����ƿ�ڻ��Թܿڣ�����ֽ����ɫ��˵�����ڰ���
��������
I.��1��t3ʱ�̸ı��������ֻ��������Ӧ���ʣ��淴Ӧ���ʲ��䣬�����ӷ�Ӧ���Ũ�ȣ���Ӧ������Ӧ������У���D�����ʵ�������t5ʱ�̣���Ӧ���淴Ӧ������У�����D�����������D�����ı仯������ͼ���е�t3��t4��
��2������ͼ��2�������¶ȵ����ߣ�C�����������С��˵��ƽ�����淴Ӧ������У�������������ԭ�����÷�Ӧ������Ӧ�����Ƿ��ȷ�Ӧ����H<0�������£�ѹǿ����C�����������������ѹǿ��ƽ��������Ӧ������У�a��b>c��d��
II.��1��10s�ڣ�����NH3�����ʵ���Ϊ1.6mol����ʱ����H2�����ʵ���Ϊ![]() =2.4mol�����ݻ�ѧ��Ӧ������ѧ����ʽ��v(H2)=
=2.4mol�����ݻ�ѧ��Ӧ������ѧ����ʽ��v(H2)=![]() =0.12mol/(L��s)���ﵽƽ��ʱ��n(NH3)=2mol��n(H2)=4mol��n(N2)=1mol������ƽ�ⳣ���ı���ʽ��K=
=0.12mol/(L��s)���ﵽƽ��ʱ��n(NH3)=2mol��n(H2)=4mol��n(N2)=1mol������ƽ�ⳣ���ı���ʽ��K=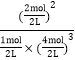 =0.25��
=0.25��
��2��NH3��Һ�������Բ��ý���ʹNH3��ΪҺ�壬��N2��H2���뿪��NH3����ѧ��ѧ����Ψһһ�ּ������壬����ʱ���Բ�����ʪ��ĺ�ɫʯ����ֽ����ƿ�ڻ��Թܿڣ�����ֽ����ɫ��˵�����ڰ�����