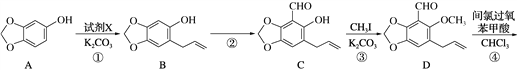
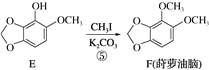
��Ŀ����
����Ŀ�����÷�Ǧ���ص�Ǧ��(PbO��Pb��PbSO4��)���Ʊ���ϸ��������Ʒ��3PbO��PbSO4��H2O (����)����Ҫ�Ʊ��������£�
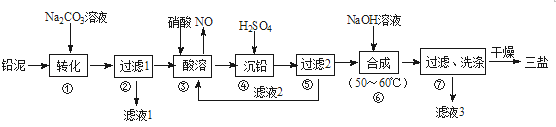
��1��������PbSO4ת��Ϊ����PbCO3�����ӷ���ʽΪ______________��
��2����Һ1����Һ3����ɫ���������ᾧ�ɵõ��ĸ���ƷΪ_________(д��ѧʽ)��
��3������������ʱ�����е�Ǧ����������Pb(NO3)2��NO�����ӷ���ʽΪ_______����Һ2��������Ҫ�ɷ�Ϊ________(д��ѧʽ)��
��4���������ϳ����εĻ�ѧ����ʽΪ___________��
��5��������ϴ�Ӳ���ʱ����������Ƿ�ϴ����ȫ�ķ�����_________��
���𰸡� CO32- +PbSO4 =PbCO3 + SO42- Na2SO410H2O����Na2SO4�� 3Pb +8H++2NO3- =3Pb2+ +2NO��+4H2O HNO3 4PbSO4+6NaOH = 3Na2SO4+3PbOPbSO4H2O+2H2O ȡ�������һ�ε�ϴ�ӹ���Һ���Թ��У������еμ������ữ��BaCl2��Һ������������ɫ�������������ϴ����ȫ
�������������������Ǧ���м���Na2CO3��Һ��PbSO4ת��Ϊ����PbCO3�����ӷ���ʽΪCO32-+PbSO4=PbCO3+SO42-��Ȼ����˵õ���Һ1ΪNa2SO4��Һ���������м����������ܣ�PbO��Pb��PbCO3�������ᷴӦ���� Pb(NO3)2��Pb�����ᷴӦ������NO��Ǧ����������Pb(NO3)2��NO�����ӷ���ʽΪ3Pb+8H++2NO3-=3Pb+2NO��+4H2O��Ȼ������Һ�м������ᣬ����bSO4���������ˣ���Һ2����Ҫ�ɷ���HNO3���������м���NaOH��Һ��������Ӧ4PbSO4+6NaOH=3Na2SO4+3PbOPbSO4H2O+2H2O������ϴ�Ӹ���õ�3PbOPbSO4H2O����Һ3�к���Na2SO4��
��1��ͨ�����Ϸ���֪��̼���ƺ�����Ǧ�����������ת�������ӷ���ʽΪCO32-+PbSO4=PbCO3+SO42-��
��2��ͨ�����Ϸ���֪����Һ1����Һ3����ɫ���������ᾧ�ɵõ��ĸ���ƷΪNa2SO410H2O(��Na2SO4)��
��3��ͨ�����Ϸ���֪�������ӷ�Ӧ����ʽΪ3Pb+8H++2NO3-=3Pb+2NO��+4H2O����Һ2����Ҫ�ɷ���δ��Ӧ��HNO3��
��4���÷�Ӧ����ʽΪ4PbSO4+6NaOH=3Na2SO4+3PbOPbSO4H2O+2H2O��
��5���ó�����������������������ӣ��������ữ���Ȼ������飬����鷽��Ϊȡ�������һ�ε�ϴ�ӹ���Һ���Թ��У������еμ������ữ��BaCl2��Һ������������ɫ�������������ϴ����ȫ��

 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�����Ŀ���Ҵ�������ϩ����ֱ��ˮ�Ϸ�����ˮ�Ϸ�������һЩת����ͼ��

ijЩ���ʵ��й��������±���
�۵�/�� | �е�/�� | ˮ���� | |
�Ҵ� | -114.1 | 78.3 | ���� |
��ȩ | -121 | 20.8 | ���� |
�������� | -83 | 77.0 | ���� |
�ش��������⣺
��1�����ˮ�Ϸ��е�ת���ۣ���ϩ��Ũ���ᷴӦ��������������(CH3CH2��OSO3H)���л���Ӧ������_____________��ת���ܵĻ�ѧ����ʽ��__________________��
��2��պ��B�IJ�����������������⻯�������ð���̣���Ӧ�Ļ�ѧ����ʽΪ________��ת���ݵĻ�ѧ����ʽΪ______________________________��
��3����һ����Ϊw��ͭ˿���ձ�ں�Ѹ�ٲ����Ҵ��У���ڵ�ͭ˿�ָ���ɫ��
��ʹͭ˿�ָ���ɫ�ķ�Ӧ�Ļ�ѧ����ʽΪ________________________��
����Ҫ֤��ͭ˿������ã�����Ҫ���еIJ�����_______________��
��4����֪��CH3CHO + NaHSO3 ��![]() ���������ǻ��һ����ƣ�������������ȩ�������·�ʽ�ᴿ��
���������ǻ��һ����ƣ�������������ȩ�������·�ʽ�ᴿ��
![]()
�٦����ǻ��һ����Ƶľ�������Ϊ___________________��
�ڷ������A��������________________��
��ijͬѧ��Ʒ������B��װ�ã��гֺͼ���װ������ȥ����ͼ��ʾ���������е�Һ��Ӧ��_____�ڽ������D����E����

�����йز�����װ�õķ�������ȷ����_____��������ĸ����ѡ���ۣ�
A���ձ���Ӧװ��ˮ
B��������Ӧͨ��ˮ
C��ͼʾװ�ÿ����ڳ�ȥ���������л��е��Ҵ�