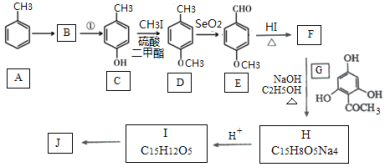
��Ŀ����
����Ŀ�������ѧ���������ɫ���������룺�Ѻ��д���CO2�Ŀ�������̼�����Һ�У��ٰ�CO2����Һ����ȡ��������ʹ֮��������Ӧ���ɿ�������Դ�״����乤��������ͼ��ʾ��
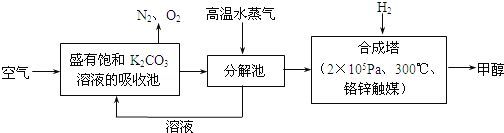
��1��д���ֽ���з�Ӧ�Ļ�ѧ����ʽΪ_______________��
��2���ںϳ����У�����4.4kg CO2������H2ǡ����ȫ��Ӧ��������̬��ˮ�ͼ״����ɷų�4947kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽ_______________��
��3����֪�ϳ����еķ�Ӧ�ǿ���ģ�����ƽ���ƶ�ԭ��������������ԭ������ת������ʵ�������в���300����¶ȣ���ԭ�������_______________��
��4������ɫ���������������г��������ʵ���ѭ����������������������������ѭ�������������ʳ�̼�����Һ�⣬������________(��ѧʽ)��
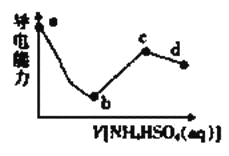
��5��300��ʱ����CO��H2��1:3������ȳ����ܱ������У�CO2��ƽ��ת����(��)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ������ͼʾ�ش��������⣺
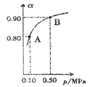
���������������䣬��A������ѹ����ԭ����һ�룬һ��ʱ���Ӧ�ٴ�ƽ���ǣ���ԭƽ��Ƚ�����˵����ȷ����________��
A��CO2��Ũ�ȼ�С |
B������Ӧ���������淴Ӧ���ʼ�С |
C��CO2��H2�������Ϊ1:3 |
D��CH3OH������������� |
�ڽ�1.0molCO2��3.0molH2�������������ܱ������У�2minʱ��Ӧ�ﵽƽ�⣬��ʱ��ϵ��ѹǿΪ0.10MPa����H2��ʾ�ķ�Ӧ����Ϊ1.2mol/(L��min)�����ܱ������������____L��
��6���״�������ȼ�ϵ�ء�������������ҺΪ����ʵĸ�����Ӧʽ��__________����ת�Ƶĵ��ӵ����ʵ���Ϊ_______molʱ���μӷ�Ӧ�������������6.72L(��״����)��
���𰸡�2KHCO3![]() K2CO3+H2O+CO2�� CO2(g)+3H2(g)�TCH3OH(g)+H2O(g)��H=-49.47kJ/mol �ӿ췴Ӧ���ʣ�ʹ�������Խϸ� ����ˮ���� CD 1 CH3OH+8OH--6e-=CO32-+6H2O 1.2
K2CO3+H2O+CO2�� CO2(g)+3H2(g)�TCH3OH(g)+H2O(g)��H=-49.47kJ/mol �ӿ췴Ӧ���ʣ�ʹ�������Խϸ� ����ˮ���� CD 1 CH3OH+8OH--6e-=CO32-+6H2O 1.2
��������
��1��̼��������ȿɷֽ��̼��ء�������̼��ˮ������ʽΪ��2KHCO3![]() K2CO3+H2O+CO2����
K2CO3+H2O+CO2����
��2������4.4 kg CO2������H2ǡ����ȫ��Ӧ���ɷų�4 947 kJ����������1 mol CO2�������ϳɼ״��ų�����49.47 kJ�������������Ȼ�ѧ����ʽΪCO2(g)+3H2(g)�TCH3OH(g)+H2O(g)��H=-49.47kJ/mol���ʴ�ΪCO2(g)+3H2(g)�TCH3OH(g)+H2O(g)��H=-49.47kJ/mol��
��3���ϳ����ڷ����ķ�ӦΪCO2(g)+3H2(g)�TCH3OH(g)+H2O(g)��H=-49.47kJ/mol������ƽ�������ƶ�������Ӧ���ʽϸߣ�ʹ�����Ļ��Խϸߣ���ͨ��ѡ��ϸ��¶��½��У�
��4��������Ӧ�����зֽ���KHCO3�ֽ����ɵ�̼��غ�ˮ�����ϳɼ״�ʱ�õ���ˮ������ѭ�����ã�
��5����A���������һ�룬CO2��Ũ��˲������Ϊԭ����ƽ�⣬ƽ��������Ӧ���ƶ���������������ԭ����ƽ���CO2��Ũ���Ա�ԭ����A����B��������٣�ѹǿ��������Ӧ���ʺ��淴Ӧ���ʾ�����B����C��CO2��H2����ʼ��Ϊ1:3���仯��Ҳ��1:3�����ƽ����������Ϊ1:3����C��ȷ��D�������С��ƽ�����������ƶ���CH3OH�������������D��ȷ����ΪCD���ڼ����������ΪVL��H2��ʾ�ķ�Ӧ����Ϊ1.2mol/(L��min)�����ݷ�Ӧ���ʵļ��㹫ʽ��֪��H2�ı仯���ʵ���Ϊ1.2mol/(L��min)��2min��VL=2.4Vmol����Ϸ�ӦCO2(g)+3H2(g)�TCH3OH(g)+H2O����CO2�仯�����ʵ�����0.8Vmol����ʱCO2ת����Ϊ0.8Vmol/1mol=0.8����V=1L��
��6���״�ȼ�ϼ��Ե���У��״��ڸ����Ϸ���������Ӧ���״�ʧ���Ӻ����������ӷ�Ӧ����̼������Ӻ�ˮ���缫��ӦʽΪCH3OH+8OH--6e-=CO32-+6H2O���μӷ�Ӧ�������������6.72L(��״����)���ʵ���Ϊ0.3mol��O2+2H2O+4e-=4OH-��ת�Ƶ������ʵ���Ϊ1.2mol��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��һ���¶��£������������Ϊ2.0 L�ĺ����ܱ������з�����Ӧ��PCl5(g)![]() PCl3(g)+Cl2(g)��
PCl3(g)+Cl2(g)��
��� | �¶� /�� | ��ʼ���� ����/mol | ƽ������ ����/mol | �ﵽƽ�� ����ʱ��/s | |
PCl5(g) | PCl3(g) | Cl2(g) | |||
�� | 320 | 0.40 | 0.10 | 0.10 | t |
�� | 320 | 0.80 | t1 | ||
�� | 410 | 0.40 | 0.15 | 0.15 | t2 |
����˵����ȷ����
A.ƽ�ⳣ��K��������>������
B.��Ӧ����ƽ��ʱ��PCl5��ת���ʣ�������>������
C.��Ӧ����ƽ��ʱ���������е�ƽ������Ϊv(PCl5)=![]() mol��L-1��s-1
mol��L-1��s-1
D.��ʼʱ���������г���PCl5 0.30 mol��PCl3 0.45 mol��Cl2 0.10 mol����Ӧ�����淴Ӧ�������
����Ŀ������˵���в���ȷ���ǣ� ��
���� | CH3COOH | HClO | H2CO3 |
����ƽ�ⳣ��(25��) | K1=1.760��10-5 | K1=2.95��10-8 | K1=4.30��10-7 K2=5.60��10-11 |
A.pH��ͬ�Ģ�CH3COONa ��NaHCO3��NaClO������Һ��c(Na+):��<��<��
B.һ��Ũ�ȵ�NaHS��Һ�У�c(Na+)+c(H+)=c(OH-)+c(HS-)+2c(S2-)
C.pH=a�İ�ˮ��ϡ����ԭ�����10������pH=b����b>a-1
D.������![]() ͨ��NaClO��Һ�з�����Ӧ��
ͨ��NaClO��Һ�з�����Ӧ��![]()
����Ŀ��ijͬѧΪ��̽��п�����ᷴӦ�����е����ʱ仯������100mLϡ�����м���������п�ۣ�����ˮ�������ռ���Ӧ�ų����������������������Ϊ��״���µ��������ʵ���¼���£��ۼ�ֵ����
ʱ��/min | 1 | 2 | 3 | 4 | 5 |
�������/mL | 50 | 120 | 232 | 290 | 310 |
��1����Ӧ��������ʱ�����__������0��1min����1��2min����2��3min������4��5min������ԭ����__��
��2����Ӧ������С��ʱ�����__������0��1min����1��2min����2��3min������4��5min������ԭ����__��
��3��2��3minʱ����ڣ��������Ũ�ȱ仯��ʾ�÷�Ӧ������Ϊ__��
��4�������Ӧ̫���ң�Ϊ�˼�����Ӧ���ʶ��ֲ����ٲ���������������ͬѧ�������зֱ��������������Һ�壬����Ϊ���е���__������ţ���
A.����ˮ B.NaCl��Һ C.Na2CO3��Һ D.CuSO4��Һ